Abstract
Objectives
The purpose of this study was to examine the functional biomechanical properties of several injectable biomaterials currently or potentially used for vocal fold augmentation.
Study Design
Rheometric investigation of phonosurgical materials in vitro.
Methods
Linear viscoelastic shear properties of 3% bovine collagen (atelocollagen), micronized Alloderm (Cymetra), calcium hydroxylapatite (CaHA) (Radiesse), and 2.4% crosslinked hyaluronic acid (HA) gel (Juvéderm) were quantified as functions of frequency covering the phonatory range, and compared to those of the human vocal fold cover. Measurements of elastic shear modulus (G′) and dynamic viscosity (η′) were made at up to 250 Hz with a controlled-strain simple-shear rheometer. Linear least-squares regression was conducted to curve-fit log G′ and log η′ versus log frequency, and statistical analysis was performed with one-way ANOVA.
Results
Radiesse and Cymetra were found to be the stiffest and the most viscous materials, followed by Juvéderm and atelocollagen. There were significant differences in the magnitudes of G′ and η′ among the phonosurgical materials and the normal human vocal fold cover (p < 0.001), whereas there was no significant difference in the frequency dependence of G′ and η′ among the materials. Post-hoc Tukey tests revealed significant differences (p < 0.05) in pairwise comparisons of the magnitudes of G′ and η′ among all materials and the vocal fold cover.
Conclusions
These findings suggested that while these biomaterials may be injected lateral to the lamina propria for the treatment of glottic insufficiency, none of them are rheologically optimal for the functional reconstruction of the vocal fold lamina propria.
Keywords: rheometry, elasticity, viscosity, injection laryngoplasty, phonosurgery
Introduction
In phonosurgery, injection laryngoplasty is an effective procedure for patients suffering from a variety of vocal pathologies. This procedure can be used for the medialization of paralyzed vocal folds for alleviating glottic insufficiency, where implantable biomaterials are usually injected lateral to the vocal fold lamina propria. This procedure can also be employed to replace, repair, or augment lamina propria deficiencies such as scarring or atrophy, where biomaterials are injected into the lamina propria.1 Vocal fold vibration depends critically on the viscoelasticity of the vocal fold lamina propria, especially the superficial layer or the cover.2, 3 Various phonosurgical biomaterials have been used for vocal fold injection, where they should be non-immunogenic, non-allergic, easy to inject, long-lasting, and have optimal viscoelastic properties in order to facilitate phonation, particularly for those materials injected into the lamina propria or cover.1–4 Despite the functional importance of the viscoelastic properties of such materials, previous studies on their viscoelasticity have been limited to measurements at low frequencies, up to only around 80 Hz for low-modulus (i.e., soft) materials.1–3, 5 Using a simple-shear rheometer capable of viscoelastic measurements at higher frequencies,6 this study aimed to provide viscoelastic data on some currently used as well as potential biomaterials at phonatory frequencies, including 3% bovine collagen (atelocollagen), micronized Alloderm (Cymetra), calcium hydroxylapatite (CaHA; Radiesse), and crosslinked hyaluronic acid (HA; Juvéderm).
Bovine collagen has had a long history of clinical use for vocal fold augmentation, including injection laryngoplasty for both medialization and the repair of focal defects.7–11 Bovine collagen can be injected with a minimally invasive procedure, and is safe and effective for the management of vocal fold paralysis.7–11 In particular, the efficacy of atelocollagen, which is 3% bovine non-crosslinked collagen treated with protease to minimize immunogenicity, has been demonstrated for vocal fold medialization.9–11 Micronized Alloderm (Cymetra) is a dermal matrix graft from donated human skin from tissue banks, rendered immunologically inert by decellularization.12 It has been used extensively for soft tissue augmentation, and has been shown to be effective for vocal fold medialization.12–14 It was found to have viscoelastic properties similar to those of bovine collagen, both in their magnitude and their changes with frequency.5 Hyaluronan or hyaluronic acid (HA) has been found to be promising for the repair of lamina propria defects, as it demonstrates near-optimal viscoelastic properties that are similar to those of the vocal fold cover.1, 3 Juvéderm is a relatively new crosslinked HA gel derived from bacterial sources and has been used as a dermal filler.15 While Juvéderm has not been reported as a vocal fold injectable, it is similar in composition to other HA gels that have been used for vocal fold augmentation, such as Restylane and Hylaform. Compared to other HA gels, Juvéderm has a higher concentration of total HA and also a higher concentration of crosslinked HA for a seemingly more long-lasting effect.16 Clinical studies using crosslinked HA gels showed that they were gradually resorbed following vocal fold injection, but to a significantly less extent than collagen.17, 18 Both collagen and HA injections resulted in significant improvements in glottal closure and perceptual voice ratings.18 Calcium hydroxylapatite (CaHA; Radiesse) has been used as an implant in a variety of applications, including the treatment of glottic insufficiency.19–21 CaHA is promising for vocal fold medialization because of its long-term stability and resistance to resorption and migration.20–22 Nonetheless, CaHA may not be suitable for the repair of lamina propria defects because its viscoelastic moduli are considerably higher than those of the vocal fold mucosa.1, 5 To gain further insights into the functional biomechanical performance of these biomaterials in the context of phonation, which occurs at frequencies of ~100–300 Hz, their linear viscoelastic shear properties were quantified as a function of frequency, up to 250 Hz.
Materials and Methods
Biomaterial samples
Viscoelastic shear properties of 2.4% crosslinked hyaluronic acid (HA) gel (Juvéderm Ultra; Allergan, Irvine, CA), micronized Alloderm (Cymetra; LifeCell Corporation, Branchburg, NJ), 3% bovine non-crosslinked collagen (Atelocollagen, Koken Co. Ltd., Tokyo, Japan) and 55.7 % calcium hydroxylapatite (CaHA) (Radiesse; Bioform Medical, San Mateo, CA) were determined as functions of frequency and compared to published values of viscoelasticity of the normal human vocal fold cover.6 HA gel samples were obtained from prepackaged, ready-to-inject syringes at a total HA concentration of 24 mg/mL (2.4%). Cymetra was in the form of dried powder of acellular human dermal matrix, and was reconstituted by saline according to manufacturer’s instructions, the same process as for clinical use. Atelocollagen samples were prepackaged in sealed ampules. Radiesse was acquired as a suspension of CaHA micropheres in a prepackaged syringe. Three samples of each material (each of around 0.1 mL) were taken and their rheometric properties were measured in a simple-shear rheometer, as described next.
Rheometric instrumentation
A custom-built, controlled-strain, linear, simple-shear rheometer system (Bose Corporation, ElectroForce Systems Group, Eden Prairie, MN) was used for viscoelastic characterization of the samples.6 As illustrated in Figure 1, a specimen was subjected to a translational, simple shear between two parallel, rectangular acrylic tissue plates. The upper plate was attached to the shaft of the linear motor through an actuator, applying a translational displacement x to the specimen at a specified magnitude and frequency. This was facilitated by displacement feedback control, with the displacement monitored by a linear variable differential transformer (LVDT). The shear force (F) resulting from the viscoelastic response of the specimen was detected by a piezoelectric force transducer attached to the lower plate. The gap size (d) of the rheometer was set to be 0.3–0.5 mm for complete contact between the specimen and the tissue plates. According to established principles for linear viscoelastic measurements,4, 6 oscillatory shear deformation of the specimen was performed at a strain amplitude of 1–2% within the linear viscoelastic region, over a frequency range of 1–250 Hz, covering phonatory frequencies. Experiments were conducted with the specimen in an environmental chamber at 37°C ± 0.1°C, with a relative humidity of close to 100 % to prevent dehydration of the specimen.
Figure 1.
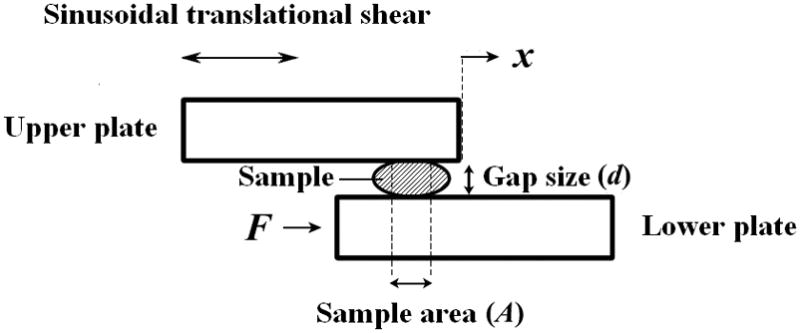
Illustration of the principle of controlled-strain, linear, simple-shear rheometry for viscoelastic measurements. A small-amplitude displacement (x) is prescribed to the sample through the upper plate, and the shear force (F) resulting from the viscoelastic response of the sample is detected through the lower plate.
The rheometer was controlled by the WINTEST software (Bose Corporation, Eden Prairie, MN), and data collection was performed on the displacement signal output of the LVDT and the force signal output of the piezoelectric force transducer, digitized at a rate of 8196 samples/s. The digitized signals were processed by the WINTEST software after the experiments, for calculating the amplitudes of the two signals and the phase shift between them. With these data, the elastic shear modulus (G′) and dynamic viscosity (η′) of the specimen were calculated as functions of frequency according to the theory of linear viscoelasticity.4, 6
Results
Figure 2 shows the elastic shear modulus (G′) of the phonosurgical biomaterials as a function of frequency, on a log-log scale as a rheological standard. Published values of G′ for the normal vocal fold cover (n = 7) according to Chan and Rodriguez, which were obtained using the same simple-shear rheometer, are also shown for comparisons.6 Figure 3 shows the dynamic viscosity (η′) of the materials, also in comparison with the normal vocal fold cover according to Chan and Rodriguez.6 The data points indicate mean values of the viscoelastic functions, with the error bars as standard deviations (only upper error bars are shown for visual clarity). Similar to the frequency dependence of the viscoelastic functions observed in previous studies,1–5 for all materials the elastic shear modulus G′ gradually increased with frequency, whereas the dynamic viscosity η′ decreased monotonically with frequency, a phenomenon known as shear thinning.4
Figure 2.
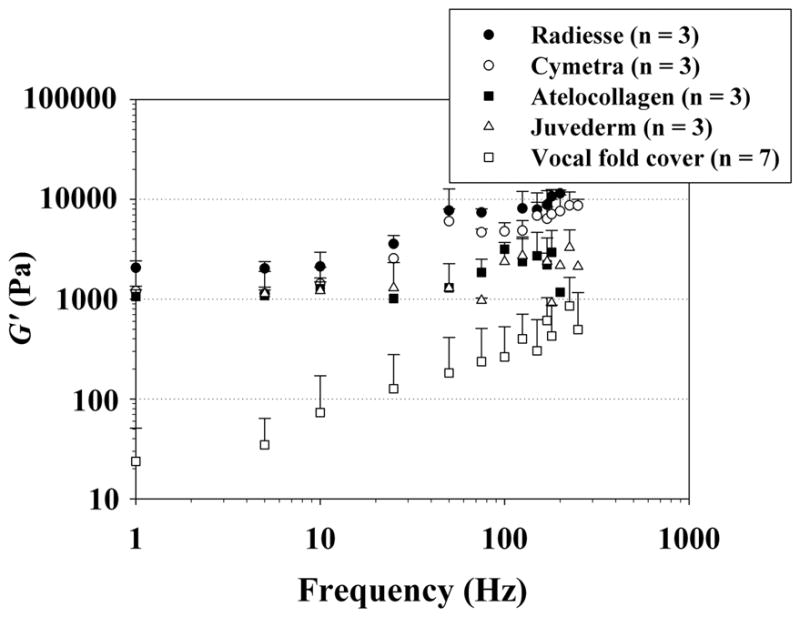
Elastic shear modulus (G′) of the injectable biomaterials (mean values + standard deviations; n = 3) and the normal human vocal fold cover (n = 7) according to Chan and Rodriguez.6
Figure 3.
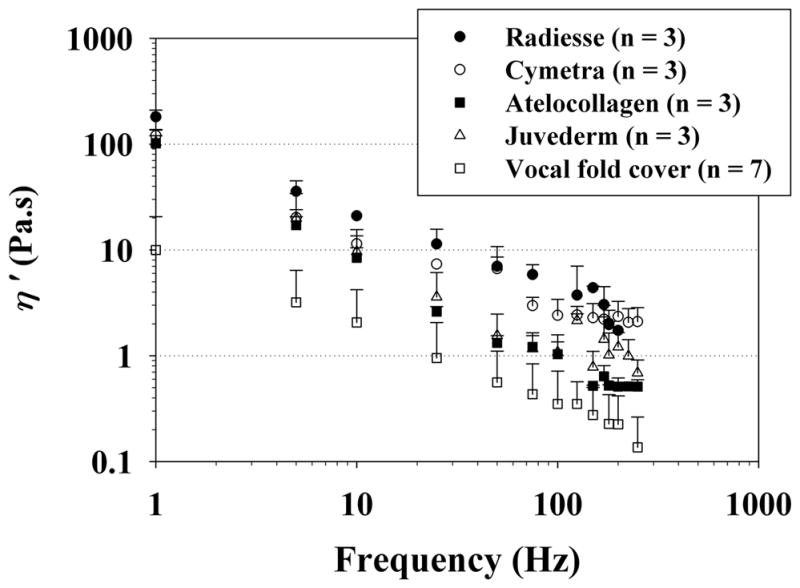
Dynamic viscosity (η′) of the injectable biomaterials (mean values + standard deviations; n = 3) and the normal human vocal fold cover (n = 7) according to Chan and Rodriguez.6
The dependence of both G′ and η′ on frequency could be parametrized by the power law relationship, as described in Chan and Rodriguez:6
| (1) |
| (2) |
where a and b are the coefficients of the parametrization, f is frequency in Hz. Linear least-squares regression analysis was performed to curve-fit the empirical data to Equations (1) and (2), with the coefficient a indicating the magnitude and the coefficient b indicating the slope on the log-log scale. Table 1 shows the results of the curve-fitting, including the coefficients a and b, and the coefficient of determination R 2 as an indication of the goodness of fit. It can be seen that G′ and η′ were well described by the power law for all materials, with high values of R 2.
Table 1.
Results of least-squares regressions for the parametric description of elastic shear modulus (G′) and dynamic viscosity (η′) of the injectable biomaterials (n = 3) and the human vocal fold cover (n = 7)6 according to Equations (1) and (2). R 2 is the coefficient of determination indicating the goodness of curve fitting.
| G′ = a f b | a (Pa.s) | b | R2 |
|---|---|---|---|
| Juvéderm (n = 3) | 721.730 | 0.219 | 0.795 |
| Cymetra (n = 3) | 827.232 | 0.407 | 0.826 |
| Atelocollagen (n = 3) | 854.108 | 0.188 | 0.775 |
| Radiesse (n = 3) | 1446.554 | 0.347 | 0.768 |
| Normal vocal fold cover (n = 7) | 13.388 | 0.698 | 0.843 |
| η′ = a f b | a (Pa.s 2) | b | R 2 |
| Juvéderm (n = 3) | 74.537 | −0.841 | 0.883 |
| Cymetra (n = 3) | 82.045 | −0.712 | 0.944 |
| Atelocollagen (n = 3) | 82.300 | −0.952 | 0.989 |
| Radiesse (n = 3) | 151.371 | −0.793 | 0.947 |
| Normal vocal fold cover (n = 7) | 8.681 | −0.709 | 0.927 |
One-way ANOVA was conducted to examine differences in the regression coefficients among the different materials and the normal vocal fold cover. Results revealed that differences in the coefficient a (the magnitude of the viscoelastic function) among the materials were statistically significant (p < 0.001) for both G′ and η′, whereas the coefficient b (the slope of the viscoelastic function) was not significantly different among the materials (Table 2). In order to determine whether each material and the normal vocal fold cover were significantly different from one another in terms of the magnitudes of G′ and η′, post-hoc pairwise comparisons with Tukey tests were performed. Results of the Tukey tests showed that there were significant differences (p < 0.05) between all materials and the normal vocal fold cover for both G′ and η′ (Table 2). In other words, all four biomaterials were significantly stiffer and more viscous than the normal vocal fold cover. Overall, Radiesse was found to have the highest magnitudes of G′ and η′ (i.e., being the stiffest and the most viscous material), followed by Cymetra. Significant differences were also found between Radiesse and Juvéderm (in both G′ and η′), between Radiesse and Cymetra (in η′), and between Radiesse and atelocollagen (in η′). No significant differences were found among Juvéderm, Cymetra, and atelocollagen in G′ and η′ (Table 2).
Table 2.
Results of one-way ANOVA for the parametric curve-fitting coefficients a and b, and post-hoc Tukey tests for pairwise comparisons of the coefficient a for elastic shear modulus (G′) and dynamic viscosity (η′) among the injectable biomaterials (n = 3) and the human vocal fold cover (n = 7).6
| Viscoelastic function | Coefficient | F (4, 14) | p value |
|---|---|---|---|
| G′ | a | 16.438 | < 0.001 |
| b | 3.375 | 0.051 | |
| η′ | a | 18.418 | < 0.001 |
| b | 2.003 | 0.149 | |
| Pairwise comparisons for G′ | p value | Pairwise comparisons for η′ | p value |
| Vocal fold cover vs. Juvéderm | 0.016 | Vocal fold cover vs. Juvéderm | 0.014 |
| Vocal fold cover vs. Cymetra | 0.006 | Vocal fold cover vs. Cymetra | 0.006 |
| Vocal fold cover vs. atelocollagen | 0.004 | Vocal fold cover vs. atelocollagen | 0.006 |
| Vocal fold cover vs. Radiesse | < 0.001 | Vocal fold cover vs. Radiesse | < 0.001 |
| Radiesse vs. Juvéderm | 0.040 | Radiesse vs. Juvéderm | 0.016 |
| Radiesse vs. Cymetra | 0.093 | Radiesse vs. Cymetra | 0.031 |
| Radiesse vs. atelocollagen | 0.115 | Radiesse vs. atelocollagen | 0.032 |
| Juvéderm vs. Cymetra | 0.989 | Juvéderm vs. Cymetra | 0.996 |
| Juvéderm vs. atelocollagen | 0.993 | Juvéderm vs. atelocollagen | 0.995 |
| Atelocollagen vs. Cymetra | 1.000 | Atelocollagen vs. Cymetra | 1.000 |
Discussion
The viscoelastic shear properties of the vocal fold lamina propria are crucial for dictating the mechanics of phonation, as the mucosal wave propagating on the vocal fold surface is a shear wave.4, 6 When the site of injection involves the lamina propria, injectable biomaterials become integrated with the tissues and influence the native viscoelastic properties of the lamina propria. For the injected vocal folds to sustain self-oscillation during phonation, the viscoelasticity of the implant should ideally match with that of the vocal fold, i.e., the injectable material should be a close rheological match with that of the injection site.1, 5 In order to achieve functional vibratory performance of the vocal fold and favorable phonatory results after the procedure, it is critical to quantify the viscoelastic shear properties of the implant materials at phonatory frequencies. Yet previous viscoelastic data reported for such materials have been limited to rather low frequencies, mostly below 80 Hz.1–3, 5 This study quantified the viscoelastic shear properties of injectable biomaterials in comparison to the normal vocal fold cover at higher frequencies, up to 250 Hz.
Least-squares regression analysis based on the power law relationship [Equations (1), (2)] could parametrize G′ and η′ reasonably well for most materials, with high values of the coefficient of determination (R 2). In rheology, the elastic shear modulus G′ is a viscoelastic function quantifying the elasticity (or energy storage) in a viscoelastic material, and it indicates the material stiffness under oscillatory shear deformation.4, 6 The dynamic viscosity η′ quantifies the viscous (energy loss) behavior of the material, and it reflects the resistance of the material to oscillatory flow.4, 6 For the magnitudes of G′ and η′, all of the injectable biomaterials were found to be at least an order of magnitude higher than those of the normal vocal fold cover (as reported by Chan and Rodriguez.6) Among the materials, Radiesse was the stiffest and the most viscous, whereas Juvéderm and atelocollagen showed the lowest shear stiffness and viscosity.
Results of ANOVA examining differences in the regression coefficients revealed similar frequency dependence for all of the phonosurgical biomaterials and the normal vocal fold cover (no significant differences in the slopes of G′ and η′), but there were significant differences in the magnitudes of G′ and η′ between all biomaterials and the normal vocal fold cover (Table 2). Post-hoc Tukey tests showed that all of the injectable materials were significantly stiffer and significantly more viscous than the normal vocal fold cover over the frequency range of 1–250 Hz (Table 2). Although Juvéderm and atelocollagen seemed to be the closest rheological match to the vocal fold cover among the materials, their G′ and η′ were significantly higher than those of the vocal fold cover. These findings suggested that none of the materials showed the kind of optimal rheological properties conducive to phonation if injected into the lamina propria.
The overall trends of the current data were comparable to those of previous reports on viscoelastic data of similar materials at lower frequencies.1–3, 5 Caton et al. reported similar values of viscoelastic properties for Radiesse and Cymetra at higher frequencies (about 150 Hz for Cymetra and about 63 Hz for Radiesse).1 The G′ and η′ of Radiesse and Cymetra were found to be more than an order of magnitude higher than those of HA gels (Restylane, Hylaform, and Carbylan-GSX 5%),1 suggesting that they are not suitable for injection involving the lamina propria, consistent with our findings. Klemuk and Titze found that Cymetra and bovine collagen (Zyderm) showed nearly identical viscoelasticity in frequency dependence and in magnitude, and their G′ and η′ were much higher than those of thiolated HA hydrogel (HA-DTPH) as well.5
As none of the present materials demonstrated rheological properties comparable to those of the human vocal fold cover, new materials with more optimal viscoelastic shear properties should be developed for injection laryngoplasty involving the vocal fold lamina propria. Some recently developed materials based on tissue engineering principles have shown promising viscoelastic properties for facilitating vocal fold vibration at phonatory frequencies.23–24
Conclusion
This study examined the rheometric properties of some commonly used and potential phonosurgical biomaterials at phonatory frequencies, including atelocollagen (3% bovine non-crosslinked collagen), Cymetra (micronized Alloderm), Radiesse (calcium hydroxylapatite), and Juvéderm (2.4% crosslinked hyaluronic acid gel). Results of linear viscoelastic measurements with a simple-shear rheometer indicated that Radiesse and Cymetra were the stiffest and the most viscous materials, whereas Juvéderm and atelocollagen showed the lowest shear stiffness and viscosity. All of the biomaterials demonstrated significantly larger magnitudes of elastic shear modulus (G′) and dynamic viscosity (η′) when compared to published values of the normal vocal fold cover at 1–250 Hz. These findings suggested that while these materials may be useful for vocal fold medialization when injected deep into the vocal fold, i.e., into the vocalis muscle, none of them are conducive to the functional vibratory performance of the vocal fold cover, and should not be used for injection into the lamina propria from a biomechanical perspective.
Acknowledgments
This study was supported by the National Institutes of Health, NIDCD Grants R01 DC006101 and R01 DC005788. The authors thank the Sankyo Foundation of Life Science in Tokyo, Japan for a postdoctoral fellowship awarded to the first author.
Footnotes
Conflict of Interest: None
References
- 1.Caton T, Thibeault SL, Klemuk S, Smith ME. Viscoelasticity of hyaluronan and nonhyaluronan based vocal fold injectables: implications for mucosal versus muscle use. Laryngoscope. 2007;117:516–521. doi: 10.1097/MLG.0b013e31802e9291. [DOI] [PubMed] [Google Scholar]
- 2.Chan RW, Titze IR. Viscosities of implantable biomaterials in vocal fold augmentation surgery. Laryngoscope. 1998;108:725–731. doi: 10.1097/00005537-199805000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Chan RW, Titze IR. Hyaluronic acid (with fibronectin) as a bioimplant for the vocal fold mucosa. Laryngoscope. 1999;109:1142–1149. doi: 10.1097/00005537-199907000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Chan RW, Titze IR. Viscoelastic shear properties of human vocal fold mucosa: measurement methodology and empirical results. J Acoust Soc Am. 1999;106:2008–2021. doi: 10.1121/1.427947. [DOI] [PubMed] [Google Scholar]
- 5.Klemuk SA, Titze IR. Viscoelastic properties of three vocal-fold injectable biomaterials at low audio frequencies. Laryngoscope. 2004;114:1597–1603. doi: 10.1097/00005537-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Chan RW, Rodriguez ML. A simple-shear rheometer for linear viscoelastic characterization of vocal fold tissues at phonatory frequencies. J Acoust Soc Am. 2008;124:1207–1219. doi: 10.1121/1.2946715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford CN. Advances and refinements in phonosurgery. Laryngoscope. 1999;109:1891–1900. doi: 10.1097/00005537-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Ford CN, Martin DW, Warner TF. Injectable collagen in laryngeal rehabilitation. Laryngoscope. 1984;94:513–518. doi: 10.1288/00005537-198404000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Kimura M, Nito T, Sakakibara K, Tayama N, Niimi S. Clinical experience with collagen injection of the vocal fold: a study of 155 patients. Auris Nasus Larynx. 2008;35:67–75. doi: 10.1016/j.anl.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Kimura M, Nito T, Imagawa H, Tayama N, Chan RW. Collagen injection as a supplement to arytenoid adduction for vocal fold paralysis. Ann Otol Rhinol Laryngol. 2008;117:430–436. doi: 10.1177/000348940811700605. [DOI] [PubMed] [Google Scholar]
- 11.Min JY, Hong SD, Kim K, Son YI. Long-term results of Artecoll injection laryngoplasty for patients with unilateral vocal fold motion impairment: safety and clinical efficacy. Arch Otolaryngol Head Neck Surg. 2008;134:490–496. doi: 10.1001/archotol.134.5.490. [DOI] [PubMed] [Google Scholar]
- 12.Pearl AW, Woo P, Ostrowski R, Mojica J, Mandell DL, Costantino P. A preliminary report on micronized AlloDerm injection laryngoplasty. Laryngoscope. 2002;112:990–996. doi: 10.1097/00005537-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Sclafani AP, Romo T, 3rd, Jacono AA, McCormick S, Cocker R, Parker A. Evaluation of acellular dermal graft in sheet (AlloDerm) and injectable (micronized AlloDerm) forms for soft tissue augmentation. Clinical observations and histological analysis. Arch Facial Plast Surg. 2000;2:130–136. doi: 10.1001/archfaci.2.2.130. [DOI] [PubMed] [Google Scholar]
- 14.Milstein CF, Akst LM, Hicks MD, Abelson TI, Strome M. Long-term effects of micronized Alloderm injection for unilateral vocal fold paralysis. Laryngoscope. 2005;115:1691–1696. doi: 10.1097/01.mlg.0000173163.07828.30. [DOI] [PubMed] [Google Scholar]
- 15.Bogdan Allemann I, Baumann L. Hyaluronic acid gel (Juvéderm) preparations in the treatment of facial wrinkles and folds. Clin Interv Aging. 2008;3:629–634. doi: 10.2147/cia.s3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumann LS, Shamban AT, Lupo MP, et al. JUVEDERM vs. ZYPLAST Nasolabial Fold Study Group. Comparison of smooth-gel hyaluronic acid dermal fillers with cross-linked bovine collagen: a multicenter, double-masked, randomized, within-subject study. Dermatol Surg. 2007;33 (Suppl 2):S128–S135. doi: 10.1111/j.1524-4725.2007.33352.x. [DOI] [PubMed] [Google Scholar]
- 17.Hertegård S, Hallen L, Laurent C, et al. Cross-linked hyaluronan used as augmentation substance for treatment of glottal insufficiency: safety aspects and vocal fold function. Laryngoscope. 2002;112:2211–2219. doi: 10.1097/00005537-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Hertegård S, Hallen L, Laurent C, et al. Cross-linked hyaluronan versus collagen for injection treatment of glottal insufficiency: 2-year follow-up. Acta Otolaryngol. 2004;124:1208–1214. doi: 10.1080/00016480410017701. [DOI] [PubMed] [Google Scholar]
- 19.Rosen CA, Thekdi AA. Vocal fold augmentation with injectable calcium hydroxylapatite: short-term results. J Voice. 2004;18:387–391. doi: 10.1016/j.jvoice.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Rosen CA, Gartner-Schmidt J, Casiano R, et al. Vocal fold augmentation with calcium hydroxylapatite (CaHA) Otolaryngol Head Neck Surg. 2007;136:198–204. doi: 10.1016/j.otohns.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Chhetri DK, Jahan-Parwar B, Hart SD, Bhuta SM, Berke GS. Injection laryngoplasty with calcium hydroxylapatite gel implant in an in vivo canine model. Ann Otol Rhinol Laryngol. 2004;113:259–264. doi: 10.1177/000348940411300402. [DOI] [PubMed] [Google Scholar]
- 22.Ozudogru E, Cakli H, Asan E, et al. The neocartilaginous formation with hydroxyl-apatite in injection laryngoplasty: an experimental study on rabbit model. Eur Arch Otorhinolaryngol. 2008;265:199–202. doi: 10.1007/s00405-007-0447-1. [DOI] [PubMed] [Google Scholar]
- 23.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich GD. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng. 2006;12:2171–2180. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- 24.Xu CC, Chan RW, Tirunagari N. A biodegradable, acellular xenogeneic scaffold for regeneration of the vocal fold lamina propria. Tissue Eng. 2007;13:551–566. doi: 10.1089/ten.2006.0169. [DOI] [PubMed] [Google Scholar]


