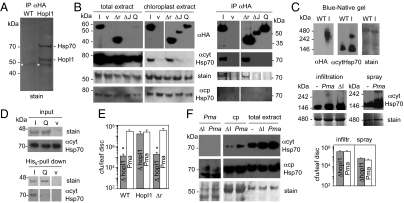
Fig. 3.
HopI1 forms complexes with Hsp70 in planta and induces Hsp70 levels. (A) Hsp70 is a major interactor of HopI1 in planta. Proteins were immunoprecipitated with anti-HA matrix from control (WT) and HopI1Pma-HA-expressing Arabidopsis, separated by SDS/PAGE and stained with Coomassie blue. Experiment was repeated with plants infected with PmaΔhopI1 with the same results. Strong bands were identified by LC-MS/MS as Hsp70 isoforms and HopI1. *, not specific (antibody). (B) J domain and HPD loop are necessary for HopI1 interaction with Hsp70. P/Q repeats of HopI1 are dispensable for this interaction. (Left) Western blots with HA antibody show that chloroplast-enriched fractions from transgenic Arabidopsis were also enriched in HopI1Pma-HA variants (I, HopI1; Δr, Δrepeats; ΔJ, ΔJ domain; Q, HPD/QAA mutant; v, vector control plants) comparing with total extracts. Plants expressing HopI1 and Δrepeats variants had elevated levels of cytHsp70, especially associated with chloroplasts (quantification is shown in Fig. S4B), whereas levels of chloroplast Hsp70 isoforms were not changed. Coomassie-stained membrane (Rubisco) shows similar loading. The same membrane was incubated with cytosolic Hsp70 monoclonal antibody and later with cpHsp70 polyclonal antibody and stained with Coomassie blue; HA signals are from another membrane with the same samples. Signals for all extract samples are from one exposure of one continuous membrane. (Right) Proteins from chloroplast-enriched extracts were immunoprecipitated with anti-HA matrix (due to uneven accumulation of HopI1-HA variants, twice more plant extract was used for IP of QAA and ΔJ than for HopI1 and Δr), separated by SDS/PAGE and stained with Coomassie blue or detected by HA antibody or cytosolic Hsp70 antibody (in separate gels/membranes). cytHsp70 precipitated with HopI1 and Δrepeats. cpHsp70 was not detected with cpHsp70 antibody in IP. Signals for all IP samples are from one exposure of one continuous membrane. IPs and immunoanalyses were repeated at least twice each with transgenic Arabidopsis plants and different HopI1 variants transiently expressed in N. benthamiana (Fig. S2), with the same results. LC-MS/MS analysis was done for two independent IPs from Arabidopsis total extracts and one from chloroplasts. (C) HopI1 and Hsp70 form high molecular mass complexes in planta. Blue-native gel of protein leaf extracts from WT and HopI1-HA (I)-expressing Arabidopsis shows HopI1 in 240–480 kDa complexes (Top). In plants expressing HopI1, Hsp70 is recruited to such high molecular mass complexes (300–350 kDa), larger than Hsp70 complexes in WT plants. The same membrane was incubated with HA antibody, and later with cytosolic Hsp70 antibody and stained with Coomassie blue. Two-dimensional gels of the same samples in Fig. S3 show that signals are from proteins of correct sizes. Large cytHsp70 complexes of similar size as in HopI1-expressing plants also formed in plants infected with Pma 1 d after infiltration at OD600 = 0.01 or spraying at OD600 = 0.1 (Middle). Levels of Pma and PmaΔhopI1 (ΔI) bacteria were similar 1 dpi (P > 0.3; Bottom). –, uninfected plants. Signals for infiltrated plants are from one exposure of one membrane and for sprayed plants from another membrane. (D) Interaction with Hsp70 depends on HopI1’s HPD loop. Recombinant His6-HopI1Psy (I) and HPD/QAA mutant of HopI1Psy (Q) were immobilized on Ni2+-NTA and incubated with Arabidopsis protein extract. Extract from E. coli transformed with empty vector was a control (v). Eluted proteins were separated by SDS/PAGE and detected with Coomassie stain (His6 proteins) and cytHsp70 antibody. Inputs are E. coli and plant extracts. Signals for all pull-down samples are from one exposure of one continuous membrane and input samples are from another membrane. Pull-down experiments were done twice with similar results. (E) P/Q repeats are necessary for virulence function of HopI1. Growth of PmaΔhopI1 strain in planta (infiltrated at OD600 = 0.0003; 3 dpi) was rescued in HopI1-expressing, but not HopI1Δrepeats-expressing Arabidopsis (protein accumulation in transgenic plants is shown in B). *P < 0.05. Growth experiments were repeated at least twice with each of two independent HopI1Δrepeats transgenic lines, giving similar results. (F) HopI1 induces and recruits Hsp70 to chloroplasts during infection. Cytosolic Hsp70 was induced by infection and recruited to chloroplasts to a greater extent when Pma harbored the HopI1 effector. Chloroplast-resident cpHsp70 levels were unaffected by infection. The same membrane was incubated with cytosolic Hsp70 monoclonal antibody and later with cpHsp70 polyclonal antibody and stained with Coomassie blue. Pma, PmaES4326 extracts showing that the cytHsp70 antibody does not recognize bacterial proteins; cp, chloroplast extracts; ΔI, PmaΔhopI1; Pma, PmaES4326; –, uninfected plants. Arabidopsis was sprayed with bacteria at OD600 = 0.1 or infiltrated at OD600 = 0.01 (shown) and Hsp70 levels were examined by Western blot analysis 1 dpi, when levels of both bacteria strains were similar (C). The average amount of cytosolic Hsp70 associated with chloroplasts was 2.2 times higher and total cytosolic Hsp70 1.5 times higher in plants infected with WT Pma than PmaΔhopI1 (Fig. S4B). At least six independent samples in two or more experiments were evaluated. Signals for all samples are from one exposure of one continuous membrane.