Figure 1.
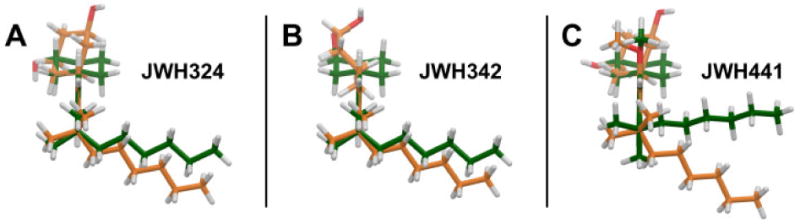
A comparison of the global minimum energy conformation (green) vs. the docked conformation (orange) of JWH-324(20), JWH-342(22) and JWH-441(8) at CB2 R* is illustrated here. The conformers have been overlaid on their phenyl rings (Ring C) and are oriented so that the phenyl ring (Ring C) is perpendicular to the plane of the page, with the dimethylheptyl side chain closest to the viewer and the carbocyclic ring (Ring A) furthest from the viewer (A) For JWH-324(20), the C1-C1a-C10a-C10 torsion angle must shift from its global minimum position of -117° to 15°. The conformational energy difference between the two conformers 5.3 kcal/mol. (B) For JWH-342 (21), the C1-C1a-C10a-C10 torsion angle must shift from its global minimum position of -116° to -48°. The total conformational cost for the ligand to dock at CB2 is 6.5 kcal/mol. (C) For JWH-441 (8), the C1-C1a-C10a-C10 torsion angle must shift from its global minimum position of -117° to 10° and the Ring C methoxy group has to shift 106° out of plane with Ring C to avoid steric interference with L6.54, S6.58, S7.39 or the ligand's A ring. The total conformational cost for the ligand to dock at CB2 R* is therefore quite high at 10.4 kcal/mol.
