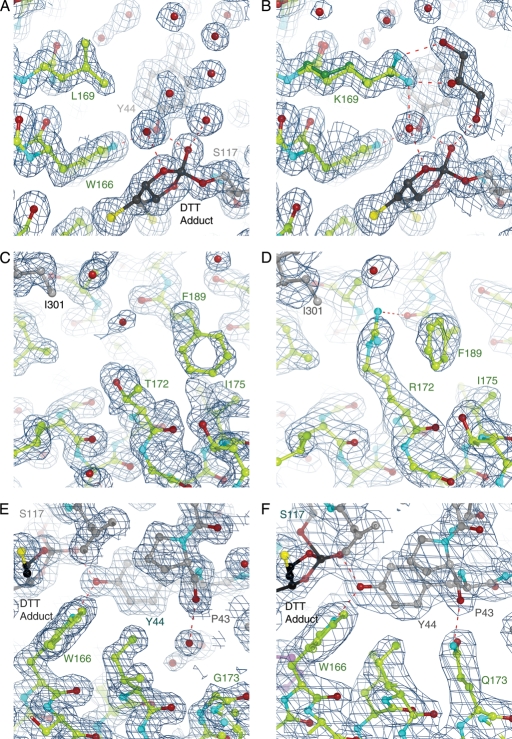
Fig. 7.
Structural analysis of stabilizing CocE mutants. The crystal structures of wt-CocE (A, C and E, respectively) compared with T172R (B), G173Q (D) and L169K (F). The stabilizing effect of the mutants appears to result from enhanced interactions between H2 and H3 of Domain II or between subunits (T172R substitution) or from additional interdomain contacts (G173Q and L169K). The L169K side chain exhibits two conformations. Note that L169 is poorly ordered in the wt-CocE structure and its side chain is solvent exposed, which is expected to be destabilizing. A DTT-carbonate adduct (DBC) is evident in all structures that contain DTT (see Supplementary data). The 2|Fo|-|Fc| electron density maps were contoured at 1 σ.