The future of sustainable energy might lie in farming the oceans, rather than in using land to grow fuel plants.
Current research aims to produce traditional biofuels from algae, but their potential to generate sustainable energy might be even greater and more ‘natural'
At the time of writing, oil continues to pour into the Gulf of Mexico. It is one of the worst environmental disasters in human history and a shocking reminder of the costs of our addiction to fossil fuels. However, the alternative sources of sustainable energy, such as wind, waves and sunshine, cannot alone replace fossil fuels in the short or even medium term. As nuclear fusion is bogged down by almost intractable engineering challenges, and nuclear fission produces toxic and radioactive waste, research has focused increasingly on converting solar energy into electricity or fuels through photosynthesis—either through the use of artificial compounds that mimic the process, or bioengineered organisms that do it ‘naturally'.
…research has focused increasingly on converting solar energy into electricity or fuels through photosynthesis…
In the ‘natural' camp, microalgae—single-celled algae—have emerged as the most promising candidates, mainly because of their potential for converting solar energy more efficiently and with less negative environmental impact than the alternatives, especially biofuel crops such as corn and soy, for example. Cyanobacteria, which are photosynthesizing prokaryotes, rather than single-celled eukaryotes, also hold promise in this regard. However, as Ben Graziano, technology commercialization manager at the Carbon Trust, an independent non-profit company set up by the UK government to develop low-carbon energy technologies, pointed out: “We may look at cyanobacteria in the future […] but they produce different co-products and we need to look at those when producing a commercial case for biofuel production.”
Perhaps surprisingly, the principal foundations of algae biofuel research were laid in the USA during the presidency of George W. Bush, particularly at the US National Renewable Energy Laboratory (NREL; Golden, CO), the largest federal agency dedicated to research on alternative energy. The interest in algae was triggered by the growing conviction that microalgae could greatly reduce the amount of land or water surface needed to produce sustainable energy, according to Mike Seibert, research fellow at NREL. “Corn grain ethanol—a current biofuel—has a solar energy conversion efficiency of about 0.05%, and thus has a huge land footprint,” he explained. “Replacing all the gasoline used in the USA with corn grain ethanol would take a corn field 1,000 miles (1,600 km) a side. Algae on the other hand have [a] theoretical conversion efficiency of 10% and in practice, 2%, and so could replace all US gasoline in an area 110 miles (176 km) a side.”
Given this promise, Europe is racing to catch up with the USA. A lobbying group, the European Algae Biomass Association (EABA), has been established with support from the European Commission to promote research and generate funding, thus demonstrating confidence that the commercial production of algae biofuels can be achieved, perhaps within as little as a decade. In the UK, the Carbon Trust has established a programme to achieve commercial-scale production of biofuels from algae by 2020. “I think by then it will have achieved parity with current biofuels, reaching US$1 per litre production costs, about 10 times cheaper than is possible with algae today,” Graziano said.
But the large-scale potential of algae biofuels remains unproven and requires more fundamental research, cautioned Pierre-Antoine Vernon, project manager of the European Biodiesel Board (EBB), a non-profit organization in Brussels, Belgium, set up in 1997 by biofuel producers to promote the development and use of biofuel in the European Union (EU). “It should be kept in mind that this is not yet a mature technology, as indicated by the diversity of algae strains, processing techniques and end products, which are typical for a nascent industry sector trying to identify the right technological path to the objective pursued,” he said.
The interest in algae was triggered by the growing conviction that microalgae could greatly reduce the amount of land or water surface needed to produce sustainable energy…
There are also regulatory and commercial factors that might inhibit large-scale deployment of algae farms for biofuel production. “You should not underestimate the regulatory barriers to the introduction of new technologies,” Vernon said. “The EBB is currently facing strong opposition from the oil and car industries in the context of the technical standardisation for biodiesel and diesel.”
Such opposition is rooted partly in the vested interests of the oil industry, but also in a natural desire to raise the bar when it comes to monitoring the safety and environmental suitability of biofuels, which must be seen to be squeaky clean and as carbon neutral as possible. “Biofuels use is under scrutiny wherever they are used, even while they represent a mere 5% of fuels used in the EU,” Vernon said. “By contrast, the remaining 95% of fossil fuels are still free from sustainability reporting, and even massive oil spills with incomparably higher consequences on biodiversity and the environment are not likely to prompt the introduction of sustainability criteria.”
There are also regulatory and commercial factors that might inhibit large-scale deployment of algae farms for biofuel production
This last point is now being put to the test by the BP spillage in the Gulf of Mexico; US President Barack Obama, in his Oval Office speech in June, called for a new focus on alternative sources of energy. Yet even this is a double-edged sword for the biofuel industry, according to Mike Griffin, an expert on the impact of oil spills from Carnegie Mellon University (Pittsburgh, PA, USA). “For the next five years you will see more money in oil spill effects work,” he said. “More money flows after each major spill until the politicians forget. This could mean less money for everything else since, with our economic situation, the pie is shrinking.”
Nevertheless, the future of algae biofuel research seems secure, even if the extent of funding depends on larger economic factors. Apart from energy conversion efficiency, algae could score from other by-products that would improve the economics of production. As Vernon noted, the economics of algae is similar to that of current biofuels in the sense that you need to find applications for the main product—the oil used to make biofuels—and the by-products, mainly protein and carbohydrates. “For soybean, which was cultivated to produce soybean meal to feed cattle long before biofuels existed, an application was already there. For algae, the challenge is to find a species whose ‘algae meal' can be used before considering biofuels production.” Promisingly, it looks as if the “algae meal” too could be used to feed animals (Becker, 2007).
In addition to protein and the oils that are used for biofuels, algae also produce carbohydrates, which could be used to produce biogas: methane and carbon dioxide. “You can recycle the CO2 back into the system, and burn the methane to produce electricity, yielding water and more CO2, which again would go back into the algae pond,” Graziano explained.
Furthermore, the conversion of lipids into biofuels can, as Vernon pointed out, be accomplished by using methods established for biodiesel production from plants. “That is one way to harness the potential of algae, as one possible feedstock for biodiesel production, by making them produce lipids that can in turn be trans-esterified into biodiesel,” he said. “Trans-esterification is a rather simple chemical reaction, for which tried-and-tested production technology emitting little greenhouse gas is available.”
There is also the more remote possibility of generating electricity directly from algae. Researchers from Stanford University in the USA and Yonsei University in Seoul, South Korea, inserted gold nanoelectrodes into individual cells, drawing one picoampere (10−12 A) of current from each (Ryu et al, 2010). At this level it would take about a trillion photosynthesizing cells more than an hour to generate the amount of energy stored in a single AA battery. Yet, as the study's lead author Won Hyoung Ryu from Yonsei University pointed out, electricity could be generated more efficiently by cutting out the intermediate step of producing biofuels, or even by creating hydrogen, for example, as a direct output—the hydrogen would still need to be burned first. “The extraction of photosynthetic electrons requires fewer energy conversion steps compared with hydrogen-based electricity production that requires at least three steps such as solar to hydrogen, hydrogen to heat, and finally heat to electricity,” Ryu said. “Every conversion step involves a certain degree of energy loss.”
But, as Ryu conceded, there are fundamental challenges to overcome: “First, we need to find a way to access the thylakoid membranes of millions of cells in parallel to obtain practically meaningful energy. Second, we still use external energy—overvoltage—to extract the photosynthetic electrons.” At present, energy to has to be put in before it can be extracted—an issue that certainly needs to be resolved if microalgae biofuels are ever to be used as constituents of self-charging batteries, for example. Doing so would also involve other challenges such as dealing with dead cells and waste products, which would have to be recycled within the battery.
Apart from energy conversion efficiency, algae could score from other by-products that would improve the economics of production
In the shorter term, microalgae will therefore be used to produce ‘traditional' biofuels, given the proven advantages of algae over land plants. According to Anastasios Melis, whose laboratory at the University of California, Berkeley, USA, specializes in microalgae, cyanobacteria and plant photosynthesis: “Proven commercial scale productivities of microalgae and cyanobacteria are much better than those of plants because of the ‘carpeting effect' […] Also, microalgae and cyanobacteria do not invest photosynthate into roots, which is biomass that cannot be harvested or exploited. There may also be secondary reasons for the efficiency advantage, such as the fact that larger plants are often limited by the supply of carbon dioxide, since their stomata tend to close under bright sunlight to protect the tissues against photo damage.”
Yet, microalgae also show reduced photosynthetic efficiency under bright sunlight. The reason is that most algal species have adapted to the low light levels below the surface of the ocean by developing large chlorophyll-based antennae for harvesting as much of the limited light available as possible. A lot of energy is then wasted under stronger sunlight because the cell is incapable of converting it all, with the rest mostly dissipated as heat. This wasteful process also mops up the incoming radiation and prevents it reaching cells at greater depths, thus further limiting the scope for the high-cell populations that are necessary to increase energy conversion.
Melis and colleagues have tackled this problem by engineering strains with shorter light-harvesting antennae by using DNA insertion mutagenesis in a model species, Chlamydomonas reinhardtii (Melis, 2007). This technique has a long history of use in gene discovery, but the sophistication required to develop algal cells that convert energy more efficiently is new. The fundamental idea is to create random mutations and identify those that generate the desired phenotype, in this case shorter light-harvesting antennae. Melis also inserted an exogenous DNA tag alongside the new base pairs, thus enabling him to locate the genomic DNA flanking the mutation. The gene affected by that mutation can then be identified as one associated with the development of light-harvesting antennae, if these are truncated in the resulting cell.
But, as with all mutations, there is a high probability that these will cause other less desirable phenotypic changes in addition to the shortened antennae. Indeed, it has turned out that such phenotypic changes often include reduced photosynthetic efficiency, thus defeating the object of the exercise. In response, Melis developed screening processes to identify those strains with truncated antennae but with fully functioning photosynthesis. This entails visually inspecting candidate colonies, as those with low densities of chlorophyll and therefore short harvesting antennae are yellowish in colour rather than green. The selected strains are then cultured and tested for energy yields during photosynthesis to identify the most efficient energy converters.
Melis has already demonstrated that cells with truncated antennae are illuminated much more uniformly in dense cultures and achieve the desired effect of creating a thick carpet of algae that efficiently harvest light. “Accordingly, the truncated light-harvesting chlorophyll antenna size property may find application in the commercial exploitation of microalgae and plants for the generation of biomass, biofuel, chemical feedstock, as well as nutraceutical and pharmaceutical products,” he said.
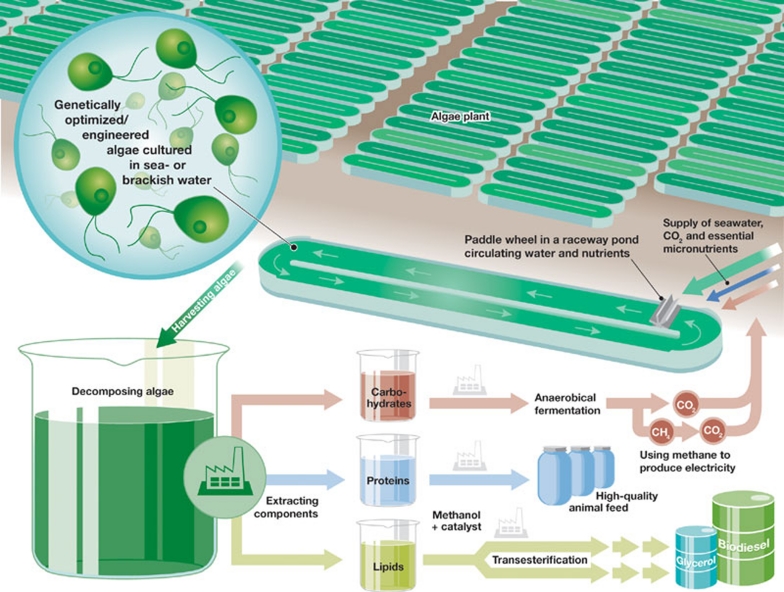
Improving the ability of algae to harvest light is an important step towards improving the efficiency of photosynthesis, especially in the densely populated volumes of water in algae biofuel farms (Fig 1). There is also the hope of going further to bioengineer microalgae to produce biofuels or electricity directly, to cut out the need to convert lipids into biofuels such as biodiesel or hydrogen. This is a harder challenge because it involves engineering a truly fundamental change in the second stage of photosynthesis—the Calvin cycle—to redirect the energy liberated by splitting water away from the normal production of glucose and towards the desired biofuel or electricity.
Figure 1.
Schematic drawing of an algae farm for the production of biofuels.
Fortunately, evolution has provided a good starting point with the hydrogenase enzyme protecting against damage when the Calvin cycle is unable to mop up all the electrons produced by the light-harvesting process. This can happen just after sunrise when light harvesting kicks in but the Calvin cycle has not yet ‘woken up' from its night's rest. Under these circumstances, hydrogenase guides the electrons directly to the protons produced by splitting water to form hydrogen. The enzyme is eventually inhibited by oxygen liberated from the Calvin cycle as it gets going to allow normal photosynthesis to resume for the day.
There is also the hope of going further to bioengineer microalgae to produce biofuels or electricity directly…
Research has therefore focused on holding back this oxygen feedback mechanism to increase production of hydrogen. The first breakthrough came in 2000 when Melis and Seibert reported that reducing sulphate levels in algal cultures would cut the rate of photosynthesis (Melis et al, 2000). The result was a 90% reduction in oxygen production, sufficient to allow the hydrogenase enzyme to continue diverting electrons towards protons to yield hydrogen for a longer period.
Although it was a considerable step forward, it did not solve the problem because the C. reinhardtii cells soon died when deprived of sulphate. Melis, Seibert and others have since worked on various methods to achieve the same effect at a molecular level without depriving the cells of sulphate ions, by diverting electrons away from the Calvin cycle while maintaining overall levels of photosynthesis. This involves getting a number of things right and will probably require tuning several genes at the whole genome level to achieve the desired objectives.
The recent announcement by Craig Venter that he has created a synthetic bacterium by transplanting the genome from another species of bacteria (Gibson et al, 2010) has therefore added a new twist to the story. Venter's technology could enable scientists to make changes to algae at the level of the whole genome, custom-building a suite of enzymatic tools to redirect the energy produced by photosynthesis. Venter's team took bacteria from the genus Mycoplasma mycoides and re-engineered its genome from digitized sequence information. The resulting genome was then transplanted into bacterial cells of another genus, Mycoplasma capricolum, which then acquired all the phenotypic properties of M. mycoides and was capable of self-replication.
Venter's development might prove a significant step on the road towards algae-derived biofuels, according to Ryu. “I think it is a smart move and look forward to hearing what would come out in the near future,” he said. “Regardless of whether it works or fails, we will always learn something. For our approach, genomic manipulation can help greatly.” A key target, Ryu explained, will be the ferredoxin proteins that act as biological capacitors in photosynthesis by accepting electrons from the chlorophyll antennae and carrying them to the Calvin cycle. “In genetically-modified algae, ferredoxin stops delivering the photosynthetic electrons to the Calvin cycle […] Then we have a better chance of stealing the electrons,” Ryu said.
Such exciting prospects stoke further optimism that science could at last provide a significant and sustainable source of energy that could be delivered in a variety of forms that might include transportation fuels, hydrogen, large-scale electricity production and possibly self-charging organic batteries.
References
- Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25: 207–210 [DOI] [PubMed] [Google Scholar]
- Gibson DG et al. (2010) Creation of a bacterial cell controlled by a chemically synthesized genome. Science 319: 1215–1220 [DOI] [PubMed] [Google Scholar]
- Melis A (2007) Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci 177: 272–280 [Google Scholar]
- Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M (2000) Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol 122: 127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu WH, Bai SJ, Park JS, Huang Z, Moseley J, Fabian T, Fasching RJ, Grossman AR, Prinz FB (2010) Direct extraction of photosynthetic electrons from single algal cells by nanoprobing system. Nano Lett 10: 1137–1143 [DOI] [PubMed] [Google Scholar]