Table 1.
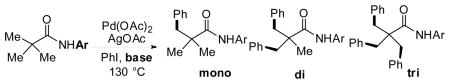 | ||||||
|---|---|---|---|---|---|---|
| Entry | Ar | base | yield (%) | |||
| sm | mono | di | tri | |||
| 1 | Ph | none | 100 | 0 | 0 | 0 |
| 2 | 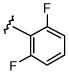 |
Cs2CO3 | 91 | 7 | 2 | 0 |
| 3 | Cs2CO3 | 39 | 41 | 12 | 8 | |
| 4 | 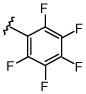 |
none | 100 | 0 | 0 | 0 |
| 5 | Cs2CO3 | 12 | 32 | 40 | 16 | |
Reaction conditions: 0.2 mmol substrate, 10 mol% Pd(OAc)2, 4 equiv AgOAc, 1.2 equiv Cs2CO3, 0.5 mL iodobenzene, 130°C,3 h, air.
Yield was determined by 1H NMR analysis of crude product using CH2Br2 as the internal standard.
