Figure 1. Examples from the cadherin superfamily.
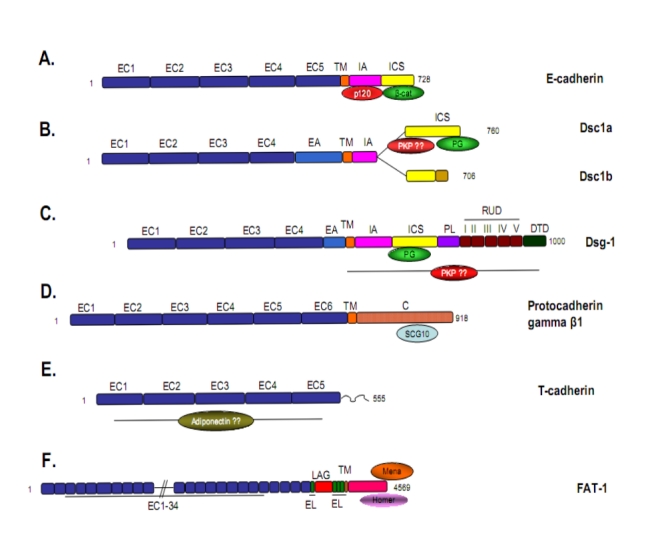
(A) The classic type I cadherin, E-cadherin, populates adherens junctions in numerous epithelial tissues, forms adhesive dimers and connects to actin and signaling pathways via beta-catenin and p120. Type II cadherins like VE-cadherin are similar but lack a HAV domain. (B) Desmocollin 1 (Dsc1) and (C) Desmoglein-1 (Dsg1) are desmosomal cadherins which resemble classic cadherins with respect to the ectodomain and the catenin binding region, however, they bind plakoglobin (Pg) and plakophilins (PKP) rather than beta-catenin and p120 and connect to intermediate filaments. Dsg1 has several unique, uncharacterized domains which extend beyond classic cadherins. Desmocollin ‘b’ differs from ‘a’ in that it has a short unique sequence at its C-terminal end and cannot bind Pg. (D) Protocadherins are heavily expressed in neural tissue and contain conserved ectodomains, but do not bind catenins. The cytoplasmic tail of gamma β-1 associates with the microtubule binding protein SCG10 and, in turn, modulates cytoskeletal dynamics [42]. (E) An atypical cadherin, T-cadherin is the only family member that lacks a transmembrane domain. Instead it associates with the plasma membrane through a GPI-anchor. An association between T-cadherin and adiponectin may contribute to carcinogenesis [43,44]. (F) Fat-1 typifies the large cadherins; although it [jam1]contains 34 ectodomains with homology to classic cadherins, it does not have a clear adhesive function but appears to modulate intracellular signaling events, particularly cytoskeletal re-organization through binding partners like Mena and Homer [45]. For a more extensive review of cadherin family members see Nollet et al. [5]. C, cytoplasmic domain; DTD, desmoglein terminal domain; EA, extracellular anchor domain; EC, extracellular cadherin domain; EL, EGF-like repeat; ICS, intracellular cadherin sequence; LAG, Laminin A G-repeat; PL, proline rich linker; RUD, repeating unit domain; TM, transmembrane.
