Figure 1.
Different MT-hTers have distinct effects on cancer cell proliferation and telomere fusion.
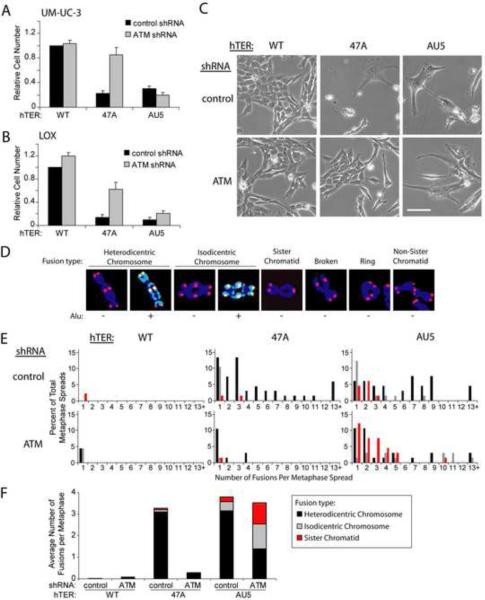
(A and B) UM-UC-3 bladder cancer (A) and LOX melanoma (B) cells were infected with lentivirus expressing the indicated shRNAs and hTERs. Cell number was determined 8 days after infection with hTER-expressing lentivirus. Cell counts were normalized to samples treated with the control shRNA and wild-type hTER. Values indicate the mean ± standard deviation of three independent hTER infections run in parallel (A) or three independent experiments (B). Rescue of proliferation of MT-hTer-47A-treated cells by prior ATM depletion was significant in both cell lines (p < 0.001 (A) and p = 0.01 (B) with the Student's t test).
(C) UM-UC-3 cells 7 days after infection with hTER-expressing lentivirus. Phase-contrast images were obtained at identical magnification; the scale bar is 100 μm.
(D) Different types of MT-hTer-induced telomere fusions in LOX cells. DAPI-stained chromosomes were labeled with probes for wild-type telomeric sequence (red) and Alu repeats (green).
(E) LOX cell metaphase spreads were prepared 5 days after infection with hTER-expressing lentivirus. Between 45 and 67 metaphase spreads for each sample were scored for the presence of the different fusion types shown in (D). For clarity, only data for chromosome-type fusions and sister chromatid fusions are shown here. Data for other fusion types are included in Fig. S1E.
(F) Average number of different telomere fusion types per metaphase.