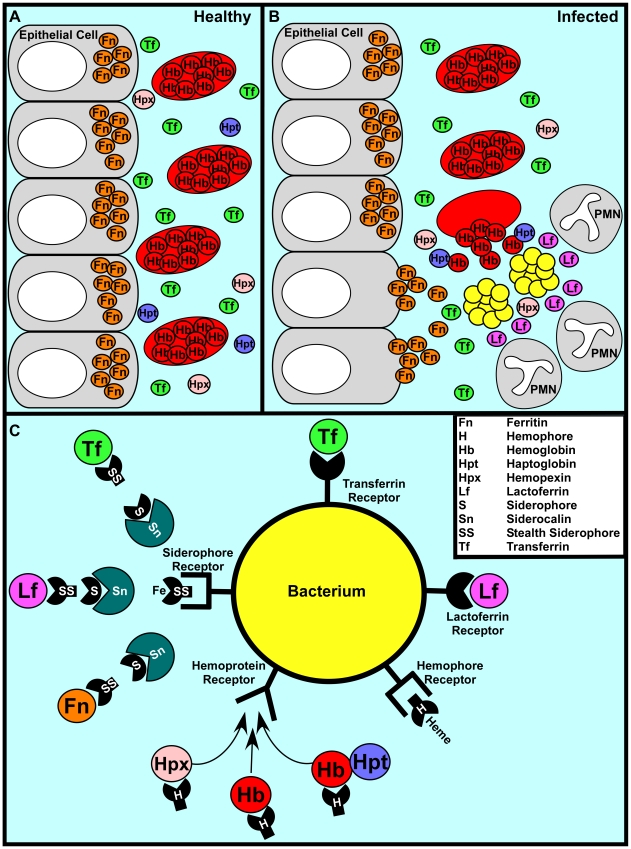
Figure 1. A representative battle during the war for iron.
(A) In a healthy individual iron is largely intracellular, sequestered within ferritin or as a cofactor of heme complexed to hemoglobin within erythrocytes. Any extracellular free iron is rapidly bound by circulating transferrin. Hemoglobin or heme that is released as a result of natural erythrocyte lysis is captured by haptoglobin and hemopexin, respectively. Taken together, these factors ensure that vertebrate tissue is virtually devoid of free iron. (B) During infection, bacterial pathogens are capable of altering the battlefield to increase the abundance of potential iron sources. Bacterial cytotoxins damage host cells, leading to the release of ferritin while hemolytic toxins lyse erythrocytes, liberating hemoglobin. The resulting inflammatory response includes the release of lactoferrin from secondary granules contained with polymorphonuclear leukocytes (PMNs). Bacterial pathogens are capable of exploiting these diverse iron sources through the elaboration of a variety of iron acquisition systems. (C) Bacterial pathogens can acquire iron through receptor-mediated recognition of transferrin, lactoferrin, hemopexin, hemoglobin, or hemoglobin–haptoglobin complexes. Alternatively, secreted siderophores can remove iron from transferrin, lactoferrin, or ferritin, whereupon siderophore–iron complexes are recognized by cognate receptors at the bacterial surface. Analogously, secreted hemophores can remove heme from hemoglobin or hemopexin and deliver heme to bacterial cells through binding with hemophore receptors. Siderophore-mediated iron acquisition is inhibited by the innate immune protein siderocalin, which binds siderophores and prevents receptor recognition. This host defense is circumvented through the production of stealth siderophores that are modified in such a way as to prevent siderocalin binding.