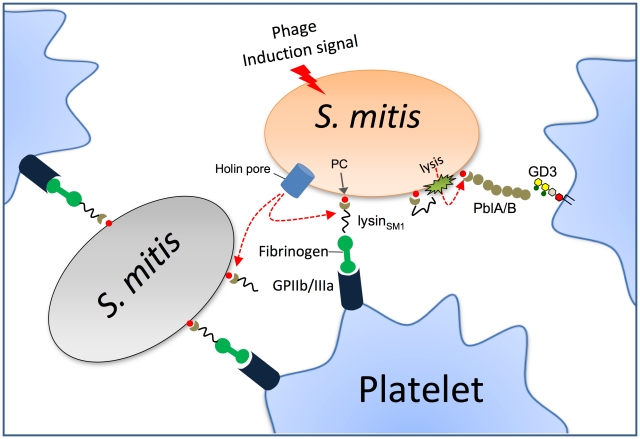
Figure 7. Model for the role of lysin in platelet binding.
Phage lysin is exported through the holin pore and mounted on the bacterial surface of the same or adjacent organisms through its interaction with PC residues. Lysin can then mediate platelet binding via its interaction with fibrinogen and glycoprotein IIb/IIIa (GPIIb/IIIa), which is the principal fibrinogen receptor on platelets. Through its amidase activity, lysin can also permeabilize the cell wall, permitting the release and surface expression PblA and PblB. These phage proteins also interact with platelets by binding the membrane ganglioside GD3.