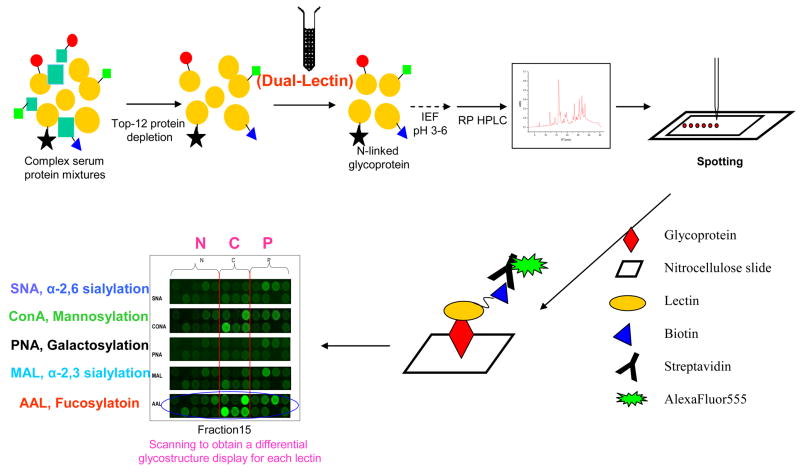
Figure 2. Experimental strategy for the study of serum glycoproteins.
Proteins in serum samples are first depleted (top-12 protein depletion) to remove abundant proteins. The sample is then enriched for N-linked glycoproteins using a dual-lectin affinity column. Glycoproteins are resolved on a non-porous silica reversed-phase high-pressure liquid chromatography (RP-HPLC) column. Resulting fractions are arrayed or spotted onto nitrocellulose slides via a non-contact piezoelectric arrayer, after which the slides are probed for specific glycan structures utilizing biotinylated lectins on a glycoprotein microarray. Scanned images of the lectin-glycoprotein interaction are visualized by secondary detection with streptavidin conjugated to a fluorescent probe. AAL aleuria aurantia, ConA concanavalin A, IEF isoelectric focusing, MAL maackia amurensis II, PNA peanut agglutinin, SNA sambucus nigra (elderberry) bark.
(Reprinted with permission from Zhao J, Patwa TH, Qiu W, Shedden K, Hinderer R, Misek DE, Anderson MA, Simeone DM, Lubman DM: Glycoprotein microarrays with multi-lectin detection: Unique lectin binding patterns as a tool for classifying normal, chronic pancreatitis and pancreatic cancer sera. J Proteome Res (2007) 6(5):1864–1874. © 2007 American Chemical Society) [53].