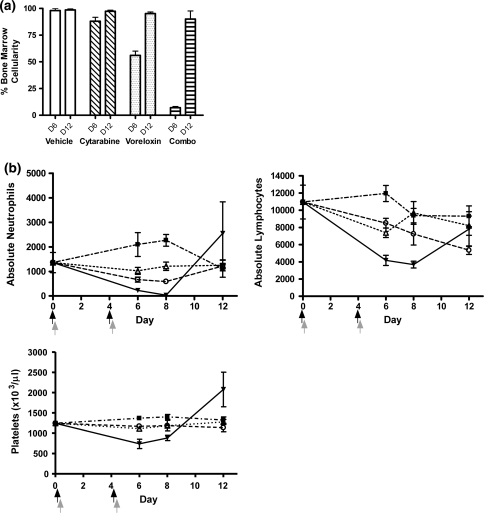
Fig. 4.
Voreloxin and cytarabine in combination causes reversible neutropenia with a more modest impact on platelets CD-1 mice received vehicle, voreloxin, cytarabine, or voreloxin and cytarabine in combination on day 0 and 4. a Percent cellularity remaining in the bone marrow on days 6 (D6) and 12 (D12): Vehicle, 0.17% methanesulfonic acid in 5% sorbitol IV q4d ×2 and water SC tid q4d ×2; Cytarabine, 20 mg/kg SC tid q4d ×2; Voreloxin, 10 mg/kg IV q4d ×2; Combo, cytarabine, 20 mg/kg SC tid q4d ×2, and voreloxin, 10 mg/kg IV q4d ×2. b Peripheral blood was isolated on days 6, 8, and 12 for analysis. Absolute neutrophils, absolute lymphocytes, and platelets (×103/μl) in circulation following treatment: (Filled square), vehicle; (Triangle), cytarabine, 20 mg/kg SC tid q4d ×2; (Circle), voreloxin, 10 mg/kg IV q4d ×2; (Filled inverted triangle), combo: cytarabine, 20 mg/kg SC tid q4d ×2, and voreloxin, 10 mg/kg IV q4d ×2. Black arrow represents the three cytarbine doses, and the gray arrow represents the voreloxin dose