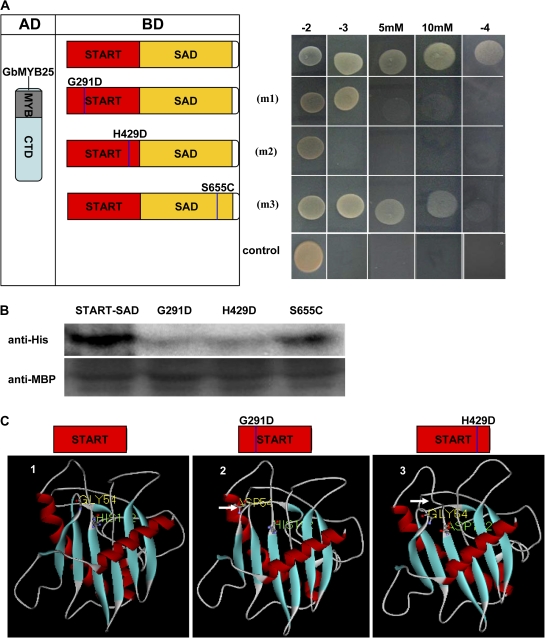
Fig. 5.
Effect of point mutations of GbML1 on protein interaction. (A) Effect of point mutations on the interaction between GbML1 and GbMYB25 in the yeast two-hybrid assay. (Left) Diagram of point mutation sites. m1 indicates a G to D mutation at the 291 site; m2 indicates an H to D mutation at the 429 site; m3 indicates an S to C mutation at the 655 amino acid site. (Right) The START–SAD domain and its mutations were fused with the BD domain and introduced into yeast with the AD-GbMYB25 construct. Yeast cells harbouring BD-START–SAD/AD were used as negative control. The growth on selective medium was shown. (B) In vitro pull-down assay to confirm the yeast two-hybrid results. MBP-GbML1-START–SAD, MBP-START–SAD-m1, MBP-START–SAD-m2, and MBP-START–SAD-m3 fusion proteins are used as baits to pull down the HIS-GbMYB25 fusion protein from the induced cell extracts. Immunoblot detection of prey protein is with the His antibody. (C) Deduced three-dimensional structure of START domains indicates the pocket structure is important for the binding. 1, Modelled structure of the native START domain from GbML1. The pocket structure is obvious on the upper side. The No. 54 amino acid (Gly) and the No. 192 amino acid (His) of the START domain are shown. 2, The G291D mutation affects the structure of the third loop. The changed conformation is shown by a white arrow. 3, The H429D mutation changes the open pocket to be closed. The white arrow indicates the changed loop.