Abstract
Antinociceptive tolerance to opioids is a well-described phenomenon, which severely limits the clinical efficacy of opioids for the treatment of chronic pain syndromes. The mechanisms that drive antinociceptive tolerance, however, are less well understood. We have previously shown that glia have a central role in the development of morphine tolerance and that administration of a glial modulating agent attenuated tolerance formation. Recently, we have demonstrated that morphine enhances microglial Iba1 expression and P2X4 receptor-mediated microglial migration via direct μ opioid receptor signaling in in vitro microglial cultures. We hypothesize that P2X4 receptors drive morphine tolerance and modulate morphine-induced spinal glial reactivity. Additionally, we hypothesize that perivascular microglia play a role in morphine tolerance and that P2X4 receptor expression regulates perivascular microglia ED2 expression. To test these hypotheses, rats were implanted with osmotic minipumps releasing morphine or saline subcutaneously for seven days. Beginning three days prior to morphine treatment, P2X4 receptor antisense oligonucleotide (asODN) was injected intrathecally daily, to selectively inhibit P2X4 receptor expression. P2X4 receptor asODN treatment inhibited morphine-induced P2X4 receptor expression and blocked antinociceptive tolerance to systemically administered morphine. P2X4 receptor asODN treatment also attenuated the morphine-dependent increase of spinal ionized calcium binding protein (Iba1), glial fibrillary acidic protein (GFAP) and μ opioid receptor protein expression. Chronic morphine also decreased perivascular microglial ED2 expression, which was reversed by P2X4 receptor asODN. Together, these data suggest that modulation of P2X4 receptor expression on microglia and perivascular microglia may prove an attractive target for adjuvant therapy to attenuate opioid-induced antinociceptive tolerance.
Keywords: opioids, purinergic, perivascular cells, microglia, astrocytes, ATP
Introduction
Opioids are the mainstay of acute pain management; however, their use in the treatment of chronic pain is limited primarily by poor efficacy, untoward side-effects and antinociceptive tolerance. Historically, opioid tolerance research has focused on neuronal mechanisms of adaptation and sensitization. Recently, our laboratory, among others, has begun to investigate the critical role of glia in morphine tolerance [30, 42].
We have previously reported that morphine administration produces robust microglial and astrocytic changes, as evidenced by increased cellular hypertrophy and microglial CD11b, Iba1 expression and astrocytic GFAP expression both in vitro [18] and in vivo [30, 32, 33, 42]. We and others have also shown that administration of the glial modulators propentofylline [33] and minocycline [8] reduced spinal CD11b and GFAP expression and attenuated antinociceptive tolerance to morphine. This suggests that glial reactivity, defined as increased glial hypertrophy, migration and release of pro-inflammatory factors, may have a critical role in the loss of antinociceptive efficacy of opioids.
Perivascular microglia are a subtype of microglia which closely appose astrocytes and endothelial cells surrounding CNS vasculature and express ED2, a molecule associated with anti-inflammatory processes [10, 56]. We have previously shown that ED2 expression is reduced following L5 spinal nerve transection and that a cannabinoid receptor 2 agonist enhanced spinal ED2 expression and inhibited tactile allodynia [36]. These results suggest that ED2 expressing perivascular microglia may have a role in the resolution of insult to the CNS.
Building upon our prior in vivo findings, we recently reported the effects of morphine on in vitro microglial migration, Iba1 and P2X4 receptor protein expression [18]. We showed that morphine enhanced P2X4 receptor-mediated microglial migration via the PI3K/Akt pathway and in a μ opioid receptor-dependent manner. It has previously been shown that P2X4 receptors play a role in chronic pain [45]. Tsuda et al. (2003) showed that pharmacological inhibition of P2X4 receptor function and asODN inhibition of P2X4 receptor expression inhibited tactile allodynia following L5 spinal nerve transection. It is now recognized that chronic neuropathic pain and morphine tolerance share many of the same features and mechanisms [24, 25, 37]; however, the role of P2X4 receptors in morphine tolerance has yet to be explored.
The study presented here was designed to investigate the role of P2X4 receptors, microglia and perivascular microglia in the development of morphine tolerance. We hypothesize that P2X4 receptors drive morphine tolerance and modulate morphine-induced spinal glial reactivity. Building upon our prior studies showing that ED2 expression in perivascular microglia is regulated following L5 spinal nerve transection, we hypothesized that perivascular microglia play a role in morphine tolerance and that P2X4 receptor expression regulates perivascular microglia ED2 expression. To test these hypotheses, intrathecal injection of asODN against P2X4 receptors was employed to inhibit receptor expression in the lumbar spinal cords of rats receiving chronic subcutaneously administered morphine. Herein, we show that P2X4 receptor asODN attenuated the development of antinociceptive tolerance to continuous morphine administration, reduced morphine-induced increases in spinal P2X4 receptor, Iba1, GFAP and μ opioid receptor expression and increased ED2 expression.
Methods
Animals
All procedures used in these studies were approved by the Dartmouth College Institutional Animal Care and Use Committee and adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain [55]. Male Sprague-Dawley rats weighing 175-200 g at the beginning of the study were housed in 12:12 hr light: dark cycle with free access to food and water. All behavioral experiments were performed in the morning between the hours of 8:00 and 11:00 am.
Osmotic Mini-Pump Implantation
Osmotic mini-pumps (ALZET Osmotic Pumps, Durect Corporation), model 2ML1 (10 μl/hr, 2 ml capacity, 8 days) were filled with saline, morphine sulfate (kind gift of the National Institute on Drug Abuse) or morphine plus naloxone (Sigma) and primed in 0.9% saline at 37°C for 16 hr prior to implantation. All morphine pumps were filled with 0.833 mg/kg/hr morphine to deliver the equivalent of twice daily 10 mg/kg injections over the course of 24 hr. Due to the constraints of pump size and animal weight, both morphine and naloxone were administered via a single pump. 0.416 mg/kg/hr naloxone was dissolved in the morphine solution to deliver the equivalent of twice daily 5 mg/kg naloxone injections over the course of 24 hr. For pump implantation, a 3 cm incision was made between the scapulae under isoflurane anesthesia and the skin was separated from the underlying fascia to create a subcutaneous pocket. Pumps were inserted with the flow regulators facing caudally and the incision was closed with surgical staples.
Intrathecal asODN Injection
The asODN sequences used here have been previously recorded [45]. P2X4 receptor asODN (5′-CAGCCCGCCATGGCTC-3′) and a mismatch asODN control (5′-ACCGCCGCCAGTCGCCT-3′) were purchased from Integrated DNA Technologies. Daily intrathecal injection of asODN was delivered via lumbar puncture. Rats were anesthetized with inhaled isoflurane anesthesia and a 25g needle was inserted into the intrathecal space between L4 and L5 lumbar vertebrae. 5 nmoles (10 μl) of either mismatch or anti-P2X4 receptor asODN was injected into the subarachnoid space via a Hamilton syringe. Positive placement of the needle was confirmed by observation of a tail flick after needle insertion and injection. Daily intrathecal injections were performed in the afternoon between 2:00 and 4:00 pm.
Mechanical and Thermal Hyperalgesia
The antinociceptive efficacy of chronic morphine and the development of antinociceptive tolerance were measured via behavioral testing of mechanical and thermal hyperalgesia. To test for mechanical hyperalgesia, paw withdrawal thresholds (g) to mechanical pressure were assessed via an Analgesia-meter (Ugo Basile), which registered the gram force weight being applied to the dorsal surface of the right hind paw. The paw withdrawal weight was registered for five trials and the mean of these trials was taken as the withdrawal threshold for the session. Animals were held by the investigator with care to induce the least stress possible. Baseline testing was completed daily for 4 days prior to osmotic pump implantations.
To test for thermal hyperalgesia, paw withdrawal latencies (s) to thermal stimulation were assessed via a Paw Thermal Stimulator with radiant heat source (8 V halogen lamp) focused on the plantar surface of the right hind paw [15]. Paw withdrawal latencies were measured with a timer that was started at the beginning of heat exposure and tripped when the paw was removed from the heat. A temperature of 54°C was used for all experiments with a cutoff of 24 s to avoid tissue damage. During each testing session, 5 trials were completed and the mean of these trials taken as the withdrawal latency for the session. Rats were acclimatized to the testing chambers for at least 30 min prior to each testing session and baseline testing was completed daily for 4 days prior to osmotic pump implantation.
Western Blot Analysis
Lumbar spinal cord (5 mm, L4-L6) tissue was harvested from 4 or 5 rats per group, placed in PBS with protease inhibitor (1:1000, Sigma), then sonicated and centrifuged. The protein in the supernatant was then quantified using the Lowry method (DC Assay, Bio-Rad). Forty micrograms of protein and a standard marker were subjected to SDS-PAGE (10% gels, Bio-Rad), transferred to PVDF membranes (Bio-Rad) and were blocked with 5% milk in TBS-Tween 20 (0.05%, Sigma). The membranes were probed with rabbit anti-P2X4 receptor primary antibody (1:250, Alomone Labs) for 16 hr at 4°C. Membranes were then incubated with goat anti-rabbit HRP-conjugated secondary antibody for 1 hr at 22°C and visualized with SuperSignal West Femto Maximum Sensitivity Substrate (Pierce) for 5 min and imaged using the Syngene G-Box (Synoptics). Membranes were stripped and reprobed with mouse monoclonal anti-β-actin primary antibody (1:1000, Abcam). Band intensities were quantified using the analysis software provided with the Syngene G-Box, as relative intensity of P2X4 receptor band divided by the intensity of the β-actin band. Data are expressed as fold change in band intensity normalized to naïve control ± SEM.
Immunofluorescence
Lumbar spinal cord (5mm, L4-L5) tissue from the remaining rats in each group (n=5) was harvested for sectioning and immunofluorescence analysis. Rats were anesthetized with isoflurane and transcardially perfused with PBS followed by 4% formaldehyde in PBS. The lumbar spinal cord was removed, postfixed with 4% formaldehyde in PBS for 2 hr and cryoprotected with 30% sucrose for 48 hr. Tissue was imbedded in OCT, frozen at −80°C and cut into 20 μm sections. Antibodies against P2X4 receptor (1:250, Calbiochem), Iba1 (1:1000, Wako), CD11b (1:250, Serotec), GFAP (1:5000, Dako), μ opioid receptor (1:5000, Neuromics), CGRP (1:500, Neuromics), NeuN (1:10,000, Chemicon) and ED2 (1:150, Serotec) were incubated with tissue sections in 1% normal goat serum and 0.01% Triton-X-100 (Sigma) for 16 hr at 4°C. The appropriate fluorescent secondary antibodies (1:250, Alexa Fluor 488 or 555) were used for each primary antibody. Non-specific staining was assessed by omitting primary antibody from the procedure. Sections for single antibody immunofluorescence were imaged with a Q-fired cooled CCD camera attached to an Olympus microscope. For quantification of Iba1, GFAP and μ opioid receptor immunofluorescence, an area encompassing the dorsal horn of the spinal cord was outlined and immunofluorescence intensities above background were quantified using SigmaScan Pro imaging analysis software as previously described [35, 36]. The total number of pixels above a predefined threshold for 6 tissue sections per animal was averaged to provide a mean fluorescence pixel value for each rat. For quantification of ED2 immunofluorescence, all ED2 positive cells were counted throughout the gray matter of 6 spinal cord sections (20 μm thick) per animal and were averaged to provide a mean number of cells per section. All data are expressed as mean (n=5 animals per group) ± SEM. For dual immunofluorescence, only primary antibodies raised in different species were used with the appropriate fluorescent secondary (rabbit anti-P2X4 receptor & mouse anti-CD11b, rabbit anti-μ opioid receptor & mouse anti-CD11b, rabbit anti-μ opioid receptor & chicken anti-CGRP, rabbit anti-μ opioid receptor & mouse anti-NeuN, rabbit anti-Iba1 & mouse anti-ED2, rabbit anti-P2X4 & mouse anti-ED2) Confocal microscopy of dual antibody immunofluorescence was performed with a Zeis LSM 510 Meta confocal microscope (Englert Cell Analysis Laboratory of Dartmouth Medical School).
Study Design
Animals were randomly divided into one of five treatment groups (n=10): naïve, P2X4 receptor asODN alone, MM asODN plus morphine, morphine alone and P2X4 receptor asODN plus morphine. An additional control group, mismatch asODN alone, was added for the immunofluorescence analyses. Beginning three days prior to pump implantation (day -3), rats in the asODN treatment groups received intrathecal injection of asODN (5 nmoles in 10ul) daily. Beginning on day 0, rats receiving morphine treatment were implanted with primed subcutaneous osmotic minipumps releasing 0.833 mg/kg/hr (2x 10 mg/kg injections per day equivalent) for seven days. Rats were tested daily (days -3 to 7) in a blinded manner for thermal and mechanical hyperalgesia behavior and were euthanized on day 7 for western blot and immunofluorescence analysis.
For the P2X4 receptor expression time course study, animals were randomly divided into one of four groups (n=4 or 5): naïve and 1, 4 or 7 days of continuous subcutaneous morphine delivered by osmotic minipump (0.833mg/kg/hr). Rats were euthanized on days 1, 4 or 7 for western blot analysis.
For the opioid receptor-dependence of morphine-induced of P2X4 receptor expression study, animals were randomly divided into one of three groups (n=4 or 5): naïve, morphine alone and morphine plus naloxone (μ opioid receptor antagonist). Beginning on day 0, rats receiving morphine treatment were implanted with primed subcutaneous osmotic minipumps releasing 0.833 mg/kg/hr (2x 10 mg/kg injections per day equivalent) for seven days. Rats receiving morphine plus naloxone treatment were implanted with primed subcutaneous osmotic minipumps co-releasing 0.833mg/kg/hr morphine (2x 10mg/kg injections per day equivalent) and 0.416 mg/kg/hr naloxone (2x 5mg/kg injections per day equivalent). Rats were tested daily on days 0, 1, 4 and 7 in a blinded manner for thermal and mechanical hyperalgesia behavior and were euthanized on day 7 for western blot analysis.
Statistical Analysis
All values are expressed as mean ± SEM. Statistical analyses were performed with GraphPad Prism 4 (GraphPad Software Inc.) with the significance level set at p<0.05. Two-way ANOVA analyses were used for all behavioral experiments and one-way ANOVA analyses were used for all western blot and immunofluorescence experiments and group differences assessed by Bonferroni post-hoc analyses.
Results
Mechanical and Thermal Hyperalgesia
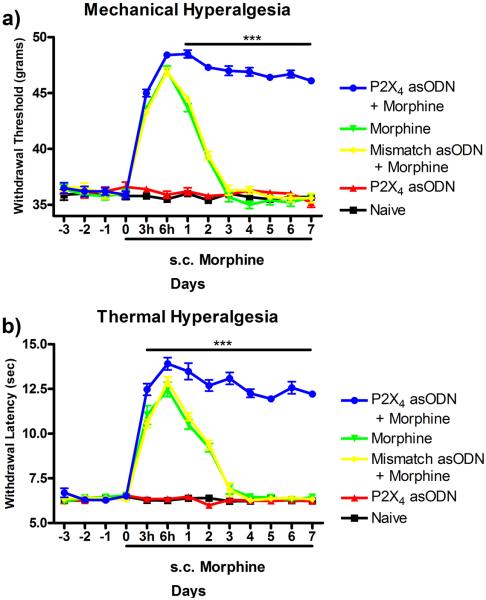
Continuous subcutaneous morphine administration produced significant acute increases in both paw withdrawal thresholds (grams) to mechanical stimulation (Fig 1a) and paw withdrawal latencies (seconds) to thermal stimulation (Fig 1b). A total loss of antinociceptive efficacy of morphine was observed in both behavioral testing paradigms by day 3 post-pump implantation. In both behavioral paradigms, daily P2X4 asODN injection maintained the efficacy of morphine and prevented the development of antinociceptive tolerance. In mechanical hyperalgesia testing (Fig 1a), morphine efficacy peaked at 6 hr post pump implantation in the morphine alone (47.0 ± 0.44g, p<0.001), mismatch asODN plus morphine (46.9 ± 0.37g, p<0.001) and P2X4 receptor asODN plus morphine (48.4 ± 0.32g, p<0.001) groups compared to naïve (35.5 ± 0.32g). By 3 days post pump implantation, the animals in the morphine alone (35.7 ± 0.45g) and mismatch asODN plus morphine (36.3 ± 0.45g) showed significant reductions in the efficacy of continuous morphine. However, daily injection of P2X4 receptor asODN maintained morphine efficacy through day 7 (46.1 ± 0.25g, p<0.001, compared to all treatment groups). In thermal hyperalgesia testing (Fig 1b), morphine efficacy also peaked at 6 hr post pump implantation (morphine alone 12.5 ± 0.43s, p<0.001; mismatch asODN plus morphine 12.9 ± 0.27s, p<0.001; P2X4 receptor asODN plus morphine 13.9 ± 0.36s, p<0.001) compared to control (naïve 6.3 ± 0.09s). A reduction of morphine antinociceptive efficacy was observed on day 3 in the morphine alone (6.9 ± 0.30s) and mismatch asODN plus morphine (6.9 ± 0.38s). Daily P2X4 receptor asODN injection maintained the efficacy of morphine and prevented antinociceptive tolerance through day 7 (12.2 ± 0.19s, p<0.001, compared to all treatment groups).
Figure 1.
Daily intrathecal injection of P2X4 receptor asODN attenuates morphine-induced antinociceptive tolerance to mechanical and thermal stimuli. Mechanical (a) and thermal (b) hyperalgesia was measured daily in all treatment groups. Morphine-induced antinociception peaked in all groups receiving chronic subcutaneous morphine peaked at 6 hr post pump implantation and antinociceptive tolerance was observed by day 3. Daily P2X4 receptor asODN maintained morphine-induced analgesia through day 7. ***p<0.001 compared to naïve, P2X4 receptor asODN alone, mismatch asODN plus morphine and morphine alone.
P2X4 Receptor Western Blot
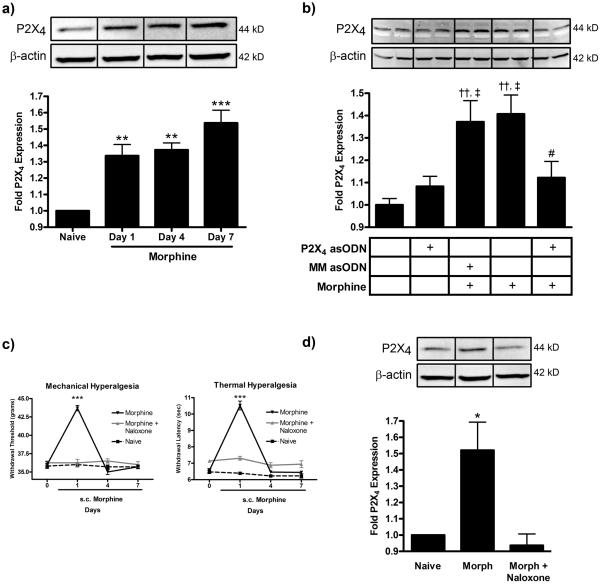
Western blot analyses were completed to determine the effect of continuous morphine treatment, daily intrathecal injection of P2X4 receptor asODN and continuous naloxone (μ opioid receptor antagonist) administration on P2X4 receptor expression (Fig 2). Experiments assessing the time course of P2X4 receptor expression revealed that continuous morphine treatment for 1, 4 or 7 days increased P2X4 receptor expression 1.34 ± 0.07 (p<0.01), 1.37 ± 0.04 (p<0.01) and 1.54 ± 0.08 (p<0.001) fold compared to naïve (Fig 2a). Experiments assessing the effects of asODN inhibition of P2X4 receptor expression similarly showed that 7 days of morphine treatment increased P2X4 receptor expression 1.37 ± 0.09 (p<0.01) and 1.41 ± 0.09 (p<0.01) fold respectively in the MM asODN plus morphine and morphine alone group compared to naïve (Fig 2b). P2X4 receptor asODN treatment reduced the morphine-induced P2X4 receptor expression to 1.12 ± 0.07 fold (p<0.05 compared to morphine alone, p>0.05 compared to naïve).
Figure 2.
P2X4 receptor asODN and naloxone attenuates morphine-induced P2X4 receptor expression. (a) Lumbar spinal cord protein from rats receiving chronic subcutaneous morphine for 0, 1, 4 or 7 days was probed for P2X4 receptor and β-actin protein expression. Quantification of fold changes of P2X4 receptor expression normalized to β-actin loading control ± SEM (n=4 or 5) is located below a representative western blot membrane. ** p<0.01, ***p<0.001 compared to naïve. (b) Day 7 lumbar spinal cord protein from naïve rats and groups receiving P2X4 receptor asODN alone, mismatch asODN plus morphine, morphine alone or P2X4 receptor asODN plus morphine was probed for P2X4 receptor and β-actin protein expression. Quantification of fold changes of P2X4 receptor expression normalized to β-actin loading control ± SEM (n=5) is located below a representative western blot membrane. †† p<0.01 compared to naïve, ‡ p<0.05 compared to P2X4 receptor asODN alone, # p<0.05 compared to morphine alone. (c) Mechanical and Thermal behavioral analgesia was measured for rats receiving subcutaneous morphine and morphine plus naloxone. ***p<0.001 compared to morphine plus naloxone and naïve. (d) Lumbar spinal cord protein from rats receiving seven days of continuous subcutaneous administration of morphine alone or morphine plus naloxone was probed for P2X4 receptor and β-actin protein expression. Quantification of fold changes of P2X4 receptor expression normalized to β-actin loading control ± SEM (n=4 or 5) is located below a representative western blot membrane. *p<0.05 compared to naïve and morphine plus naloxone.
To test if the effect of morphine on P2X4 receptor expression was opioid receptor dependent, naloxone (μ opioid receptor antagonist) was coadministered with morphine. Coadministration of naloxone blocked acute morphine analgesia in mechanical and thermal behavioral testing (Fig 2c). Naloxone also reduced morphine-induced P2X4 receptor expression from 1.52 ± 0.17 to 0.94 ± 0.07 fold (Fig 2d, p<0.05).
P2X4 Receptor and CD11b Immunofluorescence
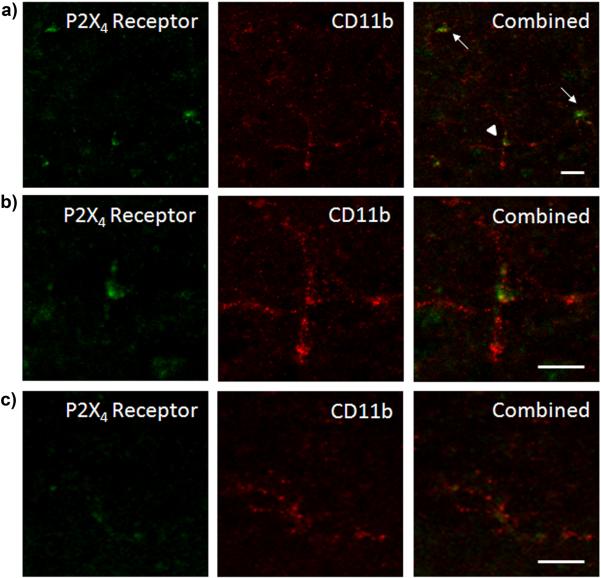
P2X4 receptor protein has previously been shown to be predominantly expressed in microglia in the rat spinal cord [12, 45, 47, 54]. We have confirmed that, in our spinal cord tissue sections P2X4 receptor staining was highly colocalized with CD11b, a marker of microglia (Fig 3). In higher magnification images of individual microglial cells from spinal cord slices of morphine tolerant rats, it was observed that P2X4 receptors were localized in clusters on the cell membrane (Fig 3b). Images from naïve rats showed less intense microglial P2X4 receptor and CD11b protein expression (Fig 3c) compared to those from morphine treated animals. We also confirmed that P2X4 receptor immunofluorescent staining did not colocalize with neurons (NeuN) or astrocytes (GFAP, data not shown).
Figure 3.
Microglia express P2X4 receptors. (a) Confocal images of P2X4 receptor (green) and CD11b (red) individually and colocalized in morphine tolerant rats. Arrows indicate cells with immunofluorescence for both P2X4 receptors and CD11b. (b) The cell highlighted by the arrow head is shown in higher magnification. (c) Confocal images of P2X4 receptor (green) and CD11b (red) individually and colocalized in naïve rats. Scale bars equal 10 μm.
Iba1 Immunofluorescence
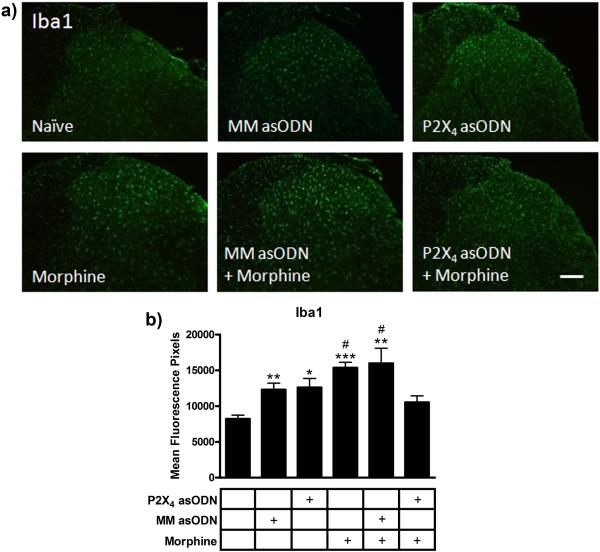
Analyses of Iba1 immunofluorescence were performed to identify microglial reactivity in the lumbar spinal cord dorsal horn across treatment groups (Fig 4). In naïve rats, staining revealed ramified microglia and Iba1 immunofluorescence of 8,205 ± 578 mean fluorescence pixels (Fig 4). After 10 days of daily intrathecal injections, the images from the P2X4 receptor asODN rats showed an increase in Iba1 immunofluorescence to 12,614 ± 1,395 mean fluorescence pixels (p<0.05 compared to naïve). To test if this was a specific response to the P2X4 receptor asODN or a non-specific response to daily intrathecal injections, an additional control group (n=4) receiving daily intrathecal injection of mismatch asODN alone was added. Analysis of this group showed a similar increase in Iba1 immunofluorescence (12,293 ± 1,063 mean fluorescence pixels, p<0.01 compared to naïve), suggesting a non-specific microglial response to intrathecal injection. The mismatch asODN plus morphine and the morphine alone group showed a nearly two fold increase in Iba1 immunofluorescence (15,975 ± 2,344 and 15,361 ± 881 mean fluorescence units respectively, p<0.01 and p<0.001 compared to naive). Daily intrathecal injection of P2X4 receptor asODN reduced the morphine-induced expression of Iba1 to 10,533 ± 996 mean fluorescence pixels (p<0.05 compared to mismatch asODN plus morphine and morphine alone groups).
Figure 4.
P2X4 receptor asODN reduced morphine-induced Iba1 expression. (a) Images of Iba1 immunofluorescence in naïve, mismatch asODN alone, P2X4 receptor asODN alone, morphine alone, mismatch asODN plus morphine and P2X4 receptor asODN plus morphine. (b) Quantification of Iba1 immunofluorescence represented as mean fluorescence pixels in the superficial dorsal horn (n=5, 5 images per animal). * p<0.05, ** p<0.01, *** p<0.001 compared to naïve. # p<0.05 compared to P2X4 receptor asODN plus morphine. Scale bars equal 50 μm.
GFAP Immunofluorescence
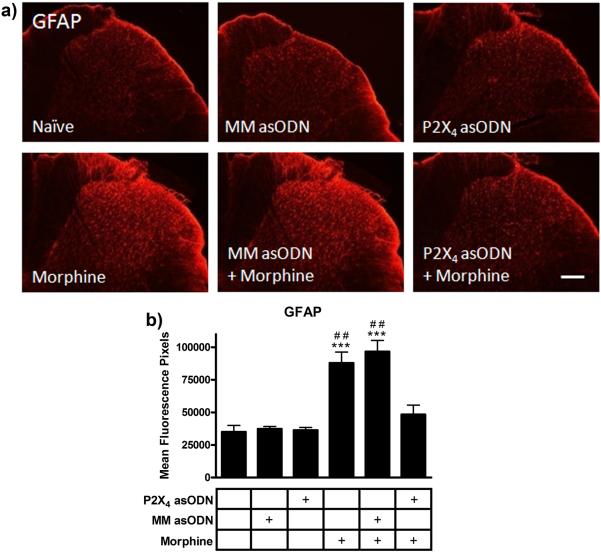
GFAP immunofluorescence staining was used to identify astrocyte reactivity in the dorsal horn following morphine and P2X4 receptor asODN administration. GFAP staining of astrocytes (Fig 5) in the mismatch asODN (37,313 ± 2,177 mean fluorescence pixels) and P2X4 receptor asODN alone (36,319 ± 2,369 mean fluorescence pixels) groups revealed no increase in expression levels compared to naïve (35,088 ± 5,503 mean fluorescence pixels, p>0.05). Chronic morphine administration robustly increased GFAP immunofluorescence in the mismatch asODN plus morphine (96,648 ± 9,508 mean fluorescence pixels, p<0.001 compared to naïve) and morphine alone groups (87,928 ± 9,359 mean fluorescence pixels, p<0.001 compared to naïve). Ten days of intrathecal injection of P2X4 receptor asODN reduced the morphine-induced expression of GFAP to naïve levels (48,363 ± 8,106 mean fluorescence pixels, p<0.01 compared to mismatch asODN plus morphine and morphine alone, p>0.05 compared to naïve).
Figure 5.
P2X4 receptor asODN reduced morphine-induced GFAP expression. (a) Images of GFAP immunofluorescence in naïve, mismatch asODN alone, P2X4 receptor asODN alone, morphine alone, mismatch asODN plus morphine and P2X4 receptor asODN plus morphine. (b) Quantification of GFAP immunofluorescence represented as mean fluorescence pixels in the superficial dorsal horn (n=5, 5 images per animal). *** p<0.001 compared to naïve. ## p<0.01 compared to P2X4 receptor asODN plus morphine. Scale bars equal 50 μm.
μ Opioid Receptor Immunofluorescence
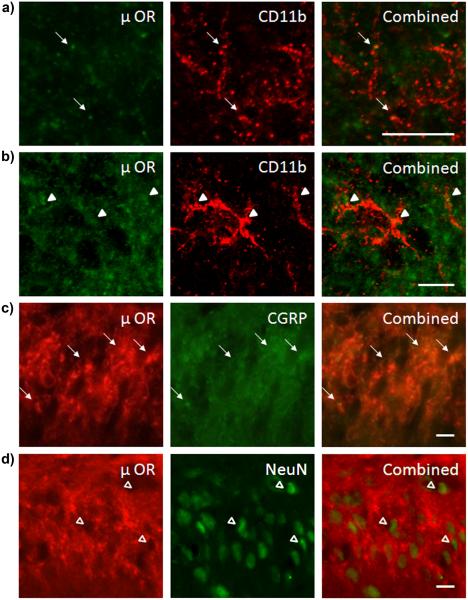
Immunofluorescence images demonstrated some μ opioid receptor colocalization with CD11b (Fig 6). Confocal images showed punctate μ opioid receptor staining on the processes (Fig 6a) and cell bodies (Fig 6b) of microglia. The majority of μ opioid receptor immunofluorescence was found in the superficial layers of the spinal cord dorsal horn and colocalized with calcitonin-related gene product protein (CGRP, Fig 6c). CGRP protein is expressed presynaptically in primary afferent C and Aδ nerve fibers arising from dorsal root ganglion neurons [11, 48, 51]. There was little evidence of colocalization between μ opioid receptors and NeuN positive spinal cord dorsal horn neurons (Fig 6d).
Figure 6.
μ opioid receptors are expressed by primary afferent C and Aδ nerve fibers and some microglia. (a) Confocal images of μ opioid receptor (green) and CD11b (red) immunofluorescence colocalization in microglial processes (arrows). (b) Confocal images of μ opioid receptor (green) and CD11b (red) immunofluorescence colocalization in microglial cell bodies (arrow heads). (c) Immunofluorescence images of μ opioid receptor (red) and CGRP (green) colocalization in afferent presynaptic nerve terminals (arrows). (d) Immunofluorescence images of μ opioid receptor (red) and NeuN (green) with no colocalization (open arrow heads). Scale bars equal 10 μm.
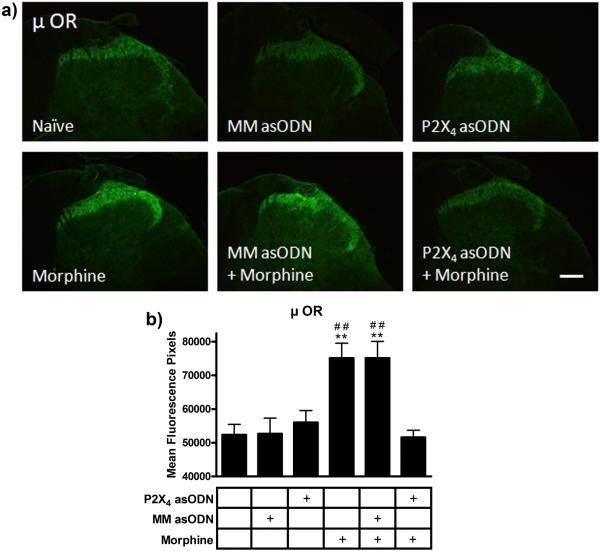
Single staining of μ opioid receptors in the superficial laminae of the dorsal horn of the lumbar spinal cord sections from each treatment group (Fig 7) showed equivalent immunofluorescence in the naïve (52,363 ± 3,464 mean fluorescence pixels) mismatch asODN (52,700 ± 5,315 mean fluorescence pixels) and P2X4 receptor asODN alone groups (56,011 ± 3,974 mean fluorescence pixels). Seven days of continuous morphine administration increased the μ opioid receptor immunofluorescence in the mismatch asODN plus morphine and morphine alone groups (75,166 ± 5,499 and 75,154 ± 5,072 mean fluorescence pixels, p<0.01 compared to naïve). Daily intrathecal injection of P2X4 receptor asODN reduced μ opioid receptor immunofluorescence in the P2X4 receptor asODN plus morphine group to naïve levels (51,591 ± 2,376 mean fluorescence pixels, p<0.01 compared to mismatch asODN plus morphine and morphine alone, p>0.05 compared to naïve).
Figure 7.
P2X4 receptor asODN reduced morphine-induced μ opioid receptor expression. (a) Images of μ opioid receptor immunofluorescence in naïve, mismatch asODN alone, P2X4 receptor asODN alone, morphine alone, mismatch asODN plus morphine and P2X4 receptor asODN plus morphine. (b) Quantification of μ opioid receptor immunofluorescence represented as mean fluorescence pixels in the superficial dorsal horn (n=5, 5 images per animal). ** p<0.01 compared to naïve. ## p<0.01 compared to P2X4 receptor asODN plus morphine. Scale bars equal 50 μm.
P2X4 Receptor and ED2 Immunofluorescence
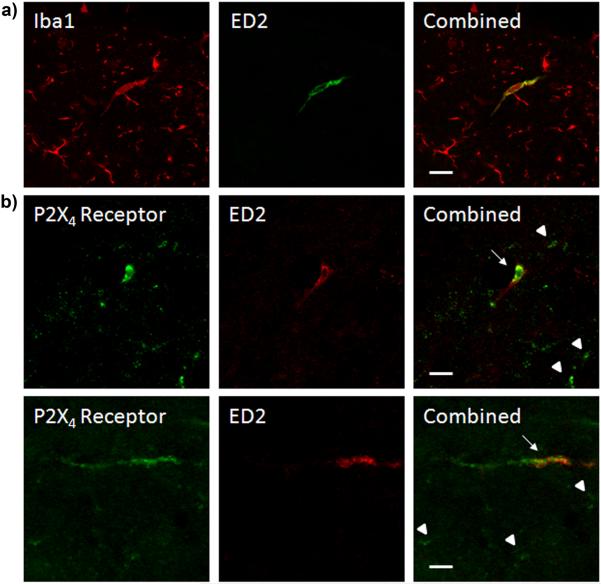
Perivascular microglial cells in the rat lumbar spinal cord express both the microglial marker Iba1 and the perivascular microglial marker ED2 (Fig 8a). Immunofluorescence colocalization revealed that ED2 positive perivascular microglia also showed marked P2X4 receptor immunoreactivity (Fig 8b). Confocal images showed that P2X4 receptor immunofluorescence was not distributed evenly across entire ED2 positive perivascular microglial cells and that P2X4 receptor positive perivascular microglia were a subset of all P2X4 receptor expressing cells.
Figure 8.
ED2 positive perivascular microglia express P2X4 receptors. (a) Confocal images of Iba1 (red) and ED2 (green) immunofluorescence individually and colocalized. (b) Confocal Images of P2X4 receptor (green) and ED2 (red) immunofluorescence individually and colocalized. Arrows indicate ED2 and P2X4 receptor expressing cells. Arrow heads indicate only P2X4 expressing cells. Scale bars equal 20 μm.
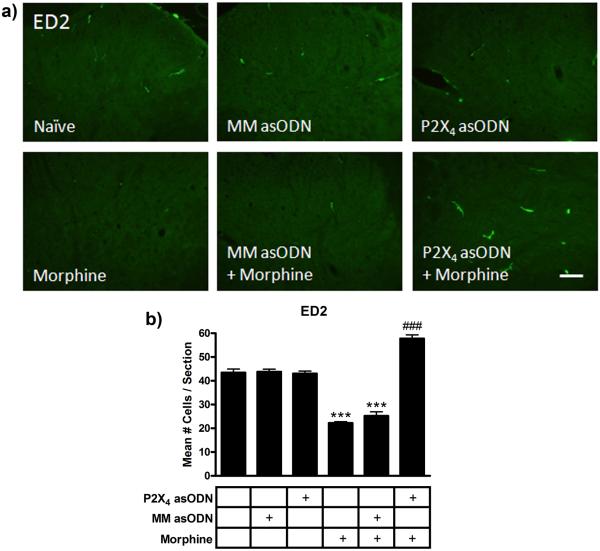
Assessment of ED2 immunofluorescence across treatment groups revealed an intriguing regulation of protein expression following morphine and asODN administration (Fig 9). The total number of ED2 positive cells in the gray matter per spinal cord section (n=6 sections/ rat) were counted and averaged across the treatment groups. In naïve rats, P2X4 receptor asODN alone and mismatch asODN alone groups, there were equivalent numbers of ED2 expressing cells in each section (43.4 ± 1.5, 43.0 ± 1.1 and 43.8 ± 1.1 cells respectively, p>0.05). In mismatch asODN plus morphine and morphine alone sections, there were significantly fewer ED2 expressing cells (25.2 ± 1.7 and 22.2 ± 0.6 cells respectively, p<0.001 compared to naïve). However, in the P2X4 receptor asODN plus morphine sections significantly more cells (57.8 ± 1.5 cells) with high immunofluorescence for ED2 were evident than in morphine treated or naïve controls (p<0.001 compared to all other treatment groups). P2X4 receptor asODN alone did not alter the number of ED2 positive cells (p>0.05), however, intrathecal injection of P2X4 receptor asODN in morphine treated animals significantly increased ED2 cells compared to naïve (p<0.001).
Figure 9.
P2X4 receptor asODN increased ED2 immunofluorescence in morphine treated animals. (a) Images of ED2 immunofluorescence in naïve, mismatch asODN alone, P2X4 receptor asODN alone, morphine alone, mismatch asODN plus morphine and P2X4 receptor asODN plus morphine. (b) Quantification of the mean number of ED2 positive cells spinal cord section (n=5, 6 × 20 μm sections per rat). *** p<0.001 compared to naïve, P2X4 asODN alone and mismatch asODN alone. ### p<0.001 compared to naïve, P2X4 receptor asODN alone, mismatch asODN alone, mismatch asODN plus morphine and morphine alone. Scale bars equal 25 μm.
Discussion
In this study we demonstrate that: (1) P2X4 receptor asODN inhibited the development of antinociceptive tolerance to chronic morphine administration; (2) P2X4 receptor asODN attenuated morphine-induced increases in P2X4 receptor, Iba1, GFAP and μ opioid receptor protein expression in the lumbar spinal cord; (3) continuous systemic morphine reduced perivascular microglial ED2 expression; (4) P2X4 receptor asODN administration enhanced perivascular microglial ED2 expression in rats receiving chronic morphine. Together, these results suggest that P2X4 receptors are critical to the reactivity state of microglia, and that perivascular microglia and play a central role in the development of morphine tolerance.
The experiments shown here are the first to investigate a role for P2X4 receptors in morphine tolerance. Microglia express several ligand gated cationic channels (P2X4,7) and G-protein coupled (P2Y2,6,12) receptors that respond to purinergic (ATP/ADP) stimulation [19]. P2X4 receptors have been shown to modulate the migration and reactivity state of microglia [17, 18, 20, 26]. Due to the lack of selective ligands, gene knockdown and knockout methodologies must be employed to selectively target P2X4 receptors. Recently, knockout studies have shown that animals lacking the P2X4 receptor gene have no deficits in pain sensitivity in non-injured naïve states. However, they show reduced tactile allodynia following L5 spinal nerve transection and intraplantar injection of complete Freund’s adjuvant [44]. This supports our findings that no changes in baseline responses to mechanical and thermal stimulation were observed in the P2X4 receptor asODN alone group, and attenuation of morphine antinociceptive tolerance was observed in the P2X4 receptor asODN plus morphine group.
The asODN sequences used in our study have been previously shown to inhibit the development of tactile allodynia following L5 spinal nerve transection [45]. Tsuda et al. (2003) observed an increase in P2X4 receptor expression following nerve injury, which was inhibited by intrathecal injection of P2X4 receptor asODN. This block of nerve injury-induced P2X4 receptor expression was associated with a reversal of tactile allodynia in L5 spinal nerve transected animals. In our study, daily intrathecal P2X4 receptor asODN injection inhibited morphine-induced expression of P2X4 receptors and blocked antinociceptive tolerance.
Microglia, the innate immune cell of the CNS, constantly survey CNS parenchyma for pathogens and cellular stress signals [14]. Our laboratory has previously shown that intrathecal catheterization alone can increase microglial reactivity as observed by enhanced CD11b immunoreactivity [9, 42]. Our data shows that ten days of asODN (either mismatch or against P2X4 receptors) induced moderate increases in Iba1 immunofluorescence, without any behavioral changes. This small increase in Iba1 immunofluorescence likely represents a non-specific response of spinal microglia to intrathecal injection. This increase in Iba1 immunofluorescence to asODN injection was not as robust as observed following morphine administration. Our laboratory has previously shown dissociation of markers of microglial reactivity and pain behaviors [7], whereby perineural administration of the local anesthetic bupivacane prior to L5 spinal nerve cryoneurolysis reduced spinal CD11b immunostaining and microglial hypertrophy, however, had no effect on tactile allodynia.
Microglia express all three subtypes of opioid receptors, μ, δ and κ [4, 46]. Studies have shown that glial and neuronal μ opioid receptors have similar morphine binding affinities, however, glia express five times fewer μ opioid receptors than neurons [23]. It is generally accepted that chronic morphine administration does not alter μ opioid receptor mRNA in the CNS [49]. However, the regulation of receptor protein is far more complex, as agonists affect μ opioid receptor expression at the posttranscriptional level via alterations in such processes as receptor phosphorylation, receptor uncoupling and receptor trafficking [1, 29]. Our findings suggest that total μ opioid receptor expression in the dorsal horn of the spinal cord may be enhanced following continuous morphine administration. This could be an adaptive response to tolerance formation, or a response to enhanced receptor internalization, because the immunofluorescence analysis completed here did not differentiate between intracellular vesicular and cell surface expressed receptors. Our experiments also show that the majority of μ opioid receptors are expressed on presynaptic afferent nerve terminals derived from dorsal root ganglion neurons and not on spinal cord dorsal horn neurons. Most studies assessing μ opioid receptor expression following morphine administration focused on neuronal receptor expression. Further studies must be completed to assess the modulation of μ opioid receptors in glia following chronic morphine administration.
Herein, we show that μ opioid receptors are expressed on both the cell bodies and processes of microglia in the dorsal horn of the lumbar spinal cord. The relatively small amount of colocalization observed by confocal microscopy between μ opioid receptors and CD11b protein indicate that enhanced receptor expression in microglia cannot account for the increase in μ opioid receptor protein observed following chronic morphine treatment. The increase in μ opioid receptor immunofluorescence is likely due to increased surface expression on primary afferent C and Aδ nerve fibers projecting from the dorsal root ganglia. Our results do indicate, however, that spinal microglia express μ opioid receptor and thus supports the hypothesis that microglia can be directly affected by opioid agonists.
In this study, astrocytes exhibited increased GFAP expression after morphine administration and reduced GFAP expression with concomitant P2X4 receptor asODN treatment. We did not observe P2X4 receptor expression on astrocytes, which suggests that inhibition of GFAP expression is downstream of a microglial process. We cannot, however, rule out that astrocytic GFAP expression is a direct effect of morphine on astrocytic opioid receptors [46] or of ATP released from neurons on astrocytic P2X or P2Y receptors [52]. Our laboratory has previously shown that minocycline, a purportedly selective microglial inhibitor, reduced astrocytic GFAP expression after L5 spinal nerve transection, further supporting the interdependency of microglial and astrocytic reactivity [31].
Perivascular microglia are antigen-presenting cells that play an important role in the immunological monitoring of the CNS and interfacing with peripheral nervous system [16, 50]. They have been shown to mediate blood borne-lymphocyte recruitment to the CNS in animal models of neuropathic pain [41] and experimental autoimmune encephalomyelitis [3]. ED2 (ED2/CD163 scavenger receptor cysteine-rich group B family member) is a specific marker for perivascular cells [13] and can be expressed on the cell surface or shed. Studies suggest that cells expressing ED2 may be anti-inflammatory in nature and that ED2 itself might play a role in the resolution of inflammation in its soluble form [10, 56].
The ED2 results presented here and shown previously by our laboratory [36] reveal that ED2 expression is regulated in a different manner than many other classical glial markers. We and others have shown that microglial CD11b or Iba1 and astrocytic GFAP expression is increased following peripheral nerve injury [42], central nerve root injury [21], peripheral surgical incision [34, 36] and chronic morphine administration [33]. Treatment with the glial modulators propentofylline and minocycline reduced tactile allodynia and decreased CD11b and GFAP expression in models of neuropathic pain [22, 43] and morphine tolerance [8, 33]. In this study, morphine treatment significantly reduced the number of ED2 positive perivascular microglia in the lumbar spinal cord. Daily intrathecal P2X4 receptor asODN administration alone had no effect on ED2 expression; however, it significantly increased the number of ED2 positive cells in morphine treated animals. Similarly, we have previously shown that peripheral nerve injury reduces ED2 expression, concomitant with behavioral sensitivity. Administration of a cannabinoid receptor 2 agonist, which inhibited tactile allodynia after nerve injury, also enhanced ED2 expression [36]. Combined with the study presented here, these data support the hypothesis that ED2 protein is anti-inflammatory in nature and might be a novel marker of glial mediated recovery from CNS injury or insult. Further studies must be undertaken to determine the mechanistic role of ED2 and perivascular microglia in the resolution of CNS insult and in the formation, maintenance and recovery from morphine tolerance.
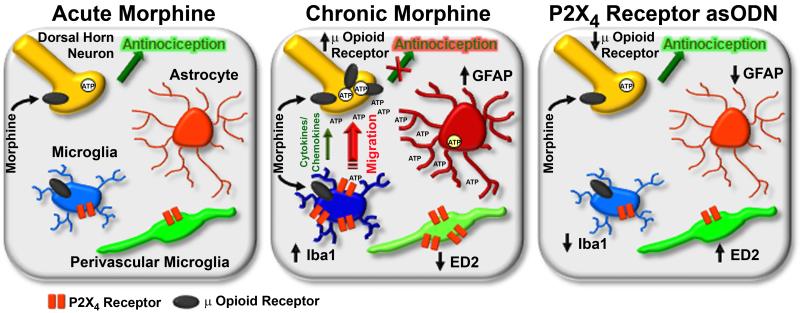
The cellular and molecular mechanisms driving morphine tolerance remain an area of intense interest. Figure 10 outlines our conceptual model of the effects of chronic morphine and P2X4 receptor asODN administration in the spinal cord. The acute antinociceptive effects of morphine are thought to be mediated through the summation of excitatory and inhibitory signaling from pre- and post-synaptic dorsal horn spinal cord neurons [5, 6] and ascending and descending pathways from supraspinal levels, including the periaqueductal gray and rostral ventromedial medulla [2, 28]. Chronically, however, morphine may induce ATP release in the spinal cord dorsal horn as a response to morphine-induced microglial reactivity. This would enhance microglial migration towards dorsal horn neurons, microglial reactivity (via P2X4 receptors) and glial release of pro-algesic factors. A positive loop of ATP release, glial reactivity and neuronal sensitization could alter the balance of inhibitory and excitatory signals from ascending and descending pathways and overcome the antinociceptive effect of morphine, leading to antinociceptive tolerance. P2X4 receptor asODN administration preserves glia in less reactive states and enhances ED2 expression, maintaining an anti-inflammatory milieu and the anti-nociceptive efficacy of morphine. Our results suggest that spinal P2X4 receptors play an integral role in morphine tolerance. Modulation of P2X4 receptors on microglia and ED2 expressing perivascular microglia may represent novel targets for the development of novel analgesics or for the attenuation of morphine tolerance.
Figure 10.
Conceptual model of the effects of chronic morphine and P2X4 receptor asODN administration on CNS cells in the spinal cord. Acutely (left panel), systemic morphine affects μ opioid receptor activation in pre and post-synaptic dorsal horn neurons in the spinal cord as well as supraspinal neurons in the periaqueductal gray and rostral ventromedial medulla. It is has been postulated that antinociception is produced as a result of the summation of direct affects on dorsal horn neurons [5, 6], inhibition of ascending nociceptive transmission and modulation of descending inhibitory and excitatory pathways [2, 28]. Chronically (center panel), morphine enhances microglial Iba1 and P2X4 receptor expression and the release of pro-algesic factors including cytokines and chemokines. In combination with reduced perivascular microglia ED2 expression, which further promotes a pro-inflammatory milieu, astrocytic GFAP expression is enhanced and neuronal sensitization develops. Spinal cord neurons are capable of releasing ATP [27, 38], which may be enhanced following sensitization. Morphine has also been shown to directly induce the release of purines from primary afferent nerve terminals [39, 40], although release of ATP has yet to be shown. ATP release from sensitized dorsal horn neurons can be propagated by astrocytes [53] to enhance P2X4 receptor-mediated migration of morphine primed microglia towards dorsal horn neurons. These reactive microglia migrate, further increasing the concentration of pro-algesic factors and enhancing neuronal sensitization. This alteration of the balance of inhibition and excitation from ascending and descending pathways reduces the efficacy of morphine, blocking antinociception. Daily intrathecal injection of P2X4 receptor asODN in the presence of morphine (right panel) reduces microglial Iba1 and astrocytic GFAP expression and enhances perivascular ED2 expression. This suppression of glial reactivity and enhanced ED2 expression may produce an anti-inflammatory milieu, preserving the antinociceptive efficacy of morphine and maintaining antinociception.
P2X4 receptor antisense oligonucleotide treatment inhibited morphine-induced increases in glial reactivity markers, enhanced perivascular microglial ED2 expression and attenuated antinociceptive tolerance to systemically administered morphine.
Acknowledgements
The authors report no conflicts of interest for the studies presented herein. The authors would like to thank the National Institute on Drug Abuse 1F30DA024933-01 (RJH) and the National Institute on Drug Abuse DA11276 and DA025987 (JAD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ammon-Treiber S, Hollt V. Morphine-induced changes of gene expression in the brain. Addict Biol. 2005;10(1):81–89. doi: 10.1080/13556210412331308994. [DOI] [PubMed] [Google Scholar]
- [2].Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- [3].Bauer J, Huitinga I, Zhao W, Lassmann H, Hickey WF, Dijkstra CD. The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia. 1995;15(4):437–446. doi: 10.1002/glia.440150407. [DOI] [PubMed] [Google Scholar]
- [4].Calvo CF, Cesselin F, Gelman M, Glowinski J. Identification of an opioid peptide secreted by rat embryonic mixed brain cells as a promoter of macrophage migration. Eur J Neurosci. 2000;12(8):2676–2684. doi: 10.1046/j.1460-9568.2000.00145.x. [DOI] [PubMed] [Google Scholar]
- [5].Chen SR, Pan HL. Blocking mu opioid receptors in the spinal cord prevents the analgesic action by subsequent systemic opioids. Brain Res. 2006;1081(1):119–125. doi: 10.1016/j.brainres.2006.01.053. [DOI] [PubMed] [Google Scholar]
- [6].Chen YP, Chen SR, Pan HL. Systemic morphine inhibits dorsal horn projection neurons through spinal cholinergic system independent of descending pathways. J Pharmacol Exp Ther. 2005;314(2):611–617. doi: 10.1124/jpet.105.085563. [DOI] [PubMed] [Google Scholar]
- [7].Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79(2):163–175. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- [8].Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ. A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun. 2008;22(1):114–123. doi: 10.1016/j.bbi.2007.07.014. [DOI] [PubMed] [Google Scholar]
- [9].DeLeo JA, Colburn RW, Rickman AJ, Yeager MP. Intrathecal catheterization alone induces neuroimmune activation in the rat. Eur J Pain. 1997;1(2):115–122. doi: 10.1016/s1090-3801(97)90069-0. [DOI] [PubMed] [Google Scholar]
- [10].Droste A, Sorg C, Hogger P. Shedding of CD163, a novel regulatory mechanism for a member of the scavenger receptor cysteine-rich family. Biochem Biophys Res Commun. 1999;256(1):110–113. doi: 10.1006/bbrc.1999.0294. [DOI] [PubMed] [Google Scholar]
- [11].Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29(13):4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gong QJ, Li YY, Xin WJ, Zang Y, Ren WJ, Wei XH, Zhang T, Liu XG. ATP induces long-term potentiation of C-fiber-evoked field potentials in spinal dorsal horn: the roles of P2X4 receptors and p38 MAPK in microglia. Glia. 2009;57(6):583–591. doi: 10.1002/glia.20786. [DOI] [PubMed] [Google Scholar]
- [13].Graeber MB, Streit WJ, Kreutzberg GW. Identity of ED2-positive perivascular cells in rat brain. J Neurosci Res. 1989;22(1):103–106. doi: 10.1002/jnr.490220114. [DOI] [PubMed] [Google Scholar]
- [14].Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- [15].Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- [16].Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239(4837):290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- [17].Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21(6):1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Horvath RJ, DeLeo JA. Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J Neurosci. 2009;29(4):998–1005. doi: 10.1523/JNEUROSCI.4595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther. 2006;109(1-2):210–226. doi: 10.1016/j.pharmthera.2005.07.001. [DOI] [PubMed] [Google Scholar]
- [20].Irino Y, Nakamura Y, Inoue K, Kohsaka S, Ohsawa K. Akt activation is involved in P2Y12 receptor-mediated chemotaxis of microglia. J Neurosci Res. 2008;86(7):1511–1519. doi: 10.1002/jnr.21610. [DOI] [PubMed] [Google Scholar]
- [21].Lacroix-Fralish ML, Tawfik VL, Tanga FY, Spratt KF, DeLeo JA. Differential spinal cord gene expression in rodent models of radicular and neuropathic pain. Anesthesiology. 2006;104(6):1283–1292. doi: 10.1097/00000542-200606000-00025. [DOI] [PubMed] [Google Scholar]
- [22].Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115(1-2):71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- [23].Maderspach K, Solomonia R. Glial and neuronal opioid receptors: apparent positive cooperativity observed in intact cultured cells. Brain Res. 1988;441(1-2):41–47. doi: 10.1016/0006-8993(88)91381-9. [DOI] [PubMed] [Google Scholar]
- [24].Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A. 1999;96(14):7731–7736. doi: 10.1073/pnas.96.14.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mika J. Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol Rep. 2008;60(3):297–307. [PubMed] [Google Scholar]
- [26].Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55(6):604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- [27].Pankratov Y, Lalo U, Verkhratsky A, North RA. Vesicular release of ATP at central synapses. Pflugers Arch. 2006;452(5):589–597. doi: 10.1007/s00424-006-0061-x. [DOI] [PubMed] [Google Scholar]
- [28].Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr., Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001;21(14):5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Raehal KM, Bohn LM. Mu opioid receptor regulation and opiate responsiveness. AAPS J. 2005;7(3):E587–591. doi: 10.1208/aapsj070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22(22):9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306(2):624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- [32].Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003;104(3):655–664. doi: 10.1016/S0304-3959(03)00138-6. [DOI] [PubMed] [Google Scholar]
- [33].Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology. 2004;29(2):327–334. doi: 10.1038/sj.npp.1300315. [DOI] [PubMed] [Google Scholar]
- [34].Romero-Sandoval A, Chai N, Nutile-McMenemy N, DeLeo JA. A Comparison of Spinal Iba1 and GFAP expression in Rodent Models of Acute and Chronic Pain. Brain Res. 2008 doi: 10.1016/j.brainres.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Romero-Sandoval A, Eisenach JC. Spinal cannabinoid receptor type 2 activation reduces hypersensitivity and spinal cord glial activation after paw incision. Anesthesiology. 2007;106(4):787–794. doi: 10.1097/01.anes.0000264765.33673.6c. [DOI] [PubMed] [Google Scholar]
- [36].Romero-Sandoval A, Nutile-McMenemy N, DeLeo JA. Spinal microglial and perivascular cell cannabinoid receptor type 2 activation reduces behavioral hypersensitivity without tolerance after peripheral nerve injury. Anesthesiology. 2008;108(4):722–734. doi: 10.1097/ALN.0b013e318167af74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Romero-Sandoval EA, Horvath RJ, DeLeo JA. Neuroimmune interactions and pain: focus on glial-modulating targets. Curr Opin Investig Drugs. 2008;9(7):726–734. [PMC free article] [PubMed] [Google Scholar]
- [38].Sawynok J, Downie JW, Reid AR, Cahill CM, White TD. ATP release from dorsal spinal cord synaptosomes: characterization and neuronal origin. Brain Res. 1993;610(1):32–38. doi: 10.1016/0006-8993(93)91213-c. [DOI] [PubMed] [Google Scholar]
- [39].Sawynok J, Sweeney MI, White TD. Adenosine release may mediate spinal analgesia by morphine. Trends Pharmacol Sci. 1989;10(5):186–189. doi: 10.1016/0165-6147(89)90235-6. [DOI] [PubMed] [Google Scholar]
- [40].Sweeney MI, White TD, Sawynok J. Morphine, capsaicin and K+ release purines from capsaicin-sensitive primary afferent nerve terminals in the spinal cord. J Pharmacol Exp Ther. 1989;248(1):447–454. [PubMed] [Google Scholar]
- [41].Sweitzer SM, Hickey WF, Rutkowski MD, Pahl JL, DeLeo JA. Focal peripheral nerve injury induces leukocyte trafficking into the central nervous system: potential relationship to neuropathic pain. Pain. 2002;100(1-2):163–170. doi: 10.1016/s0304-3959(02)00257-9. [DOI] [PubMed] [Google Scholar]
- [42].Tawfik VL, LaCroix-Fralish ML, Nutile-McMenemy N, DeLeo JA. Transcriptional and translational regulation of glial activation by morphine in a rodent model of neuropathic pain. J Pharmacol Exp Ther. 2005;313(3):1239–1247. doi: 10.1124/jpet.104.082420. [DOI] [PubMed] [Google Scholar]
- [43].Tawfik VL, Nutile-McMenemy N, Lacroix-Fralish ML, Deleo JA. Efficacy of propentofylline, a glial modulating agent, on existing mechanical allodynia following peripheral nerve injury. Brain Behav Immun. 2007;21(2):238–246. doi: 10.1016/j.bbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- [44].Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain. 2009;5:28. doi: 10.1186/1744-8069-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- [46].Turchan-Cholewo J, Dimayuga FO, Ding Q, Keller JN, Hauser KF, Knapp PE, Bruce-Keller AJ. Cell-specific actions of HIV-Tat and morphine on opioid receptor expression in glia. J Neurosci Res. 2008;86(9):2100–2110. doi: 10.1002/jnr.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28(44):11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang Z, Ma W, Chabot JG, Quirion R. Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J. 2009;23(8):2576–2586. doi: 10.1096/fj.08-128348. [DOI] [PubMed] [Google Scholar]
- [49].Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81(1):299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- [50].Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193(8):905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yu LC, Hou JF, Fu FH, Zhang YX. Roles of calcitonin gene-related peptide and its receptors in pain-related behavioral responses in the central nervous system. Neurosci Biobehav Rev. 2009;33(8):1185–1191. doi: 10.1016/j.neubiorev.2009.03.009. [DOI] [PubMed] [Google Scholar]
- [52].Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A. 2007;104(23):9864–9869. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol. 2007;9(8):945–953. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- [54].Zhang Z, Zhang ZY, Fauser U, Schluesener HJ. Mechanical allodynia and spinal up-regulation of P2X(4) receptor in experimental autoimmune neuritis rats. Neuroscience. 2008;152(2):495–501. doi: 10.1016/j.neuroscience.2007.12.042. [DOI] [PubMed] [Google Scholar]
- [55].Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- [56].Zwadlo G, Voegeli R, Osthoff KS, Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Exp Cell Biol. 1987;55(6):295–304. doi: 10.1159/000163432. [DOI] [PubMed] [Google Scholar]