Figure 10.
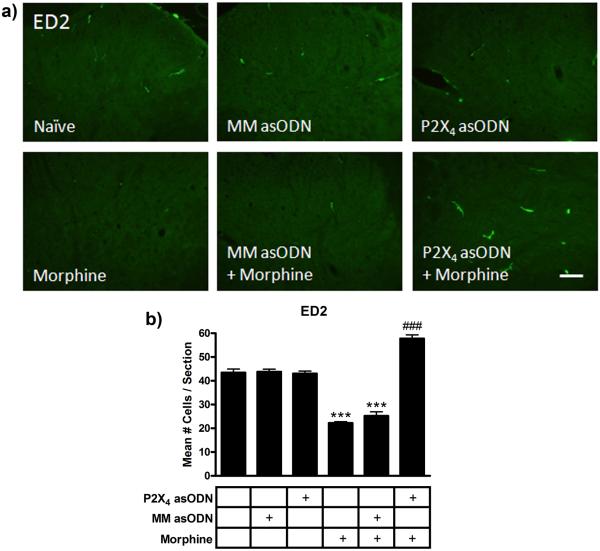
Conceptual model of the effects of chronic morphine and P2X4 receptor asODN administration on CNS cells in the spinal cord. Acutely (left panel), systemic morphine affects μ opioid receptor activation in pre and post-synaptic dorsal horn neurons in the spinal cord as well as supraspinal neurons in the periaqueductal gray and rostral ventromedial medulla. It is has been postulated that antinociception is produced as a result of the summation of direct affects on dorsal horn neurons [5, 6], inhibition of ascending nociceptive transmission and modulation of descending inhibitory and excitatory pathways [2, 28]. Chronically (center panel), morphine enhances microglial Iba1 and P2X4 receptor expression and the release of pro-algesic factors including cytokines and chemokines. In combination with reduced perivascular microglia ED2 expression, which further promotes a pro-inflammatory milieu, astrocytic GFAP expression is enhanced and neuronal sensitization develops. Spinal cord neurons are capable of releasing ATP [27, 38], which may be enhanced following sensitization. Morphine has also been shown to directly induce the release of purines from primary afferent nerve terminals [39, 40], although release of ATP has yet to be shown. ATP release from sensitized dorsal horn neurons can be propagated by astrocytes [53] to enhance P2X4 receptor-mediated migration of morphine primed microglia towards dorsal horn neurons. These reactive microglia migrate, further increasing the concentration of pro-algesic factors and enhancing neuronal sensitization. This alteration of the balance of inhibition and excitation from ascending and descending pathways reduces the efficacy of morphine, blocking antinociception. Daily intrathecal injection of P2X4 receptor asODN in the presence of morphine (right panel) reduces microglial Iba1 and astrocytic GFAP expression and enhances perivascular ED2 expression. This suppression of glial reactivity and enhanced ED2 expression may produce an anti-inflammatory milieu, preserving the antinociceptive efficacy of morphine and maintaining antinociception.