Table 1.
 | |||||
|---|---|---|---|---|---|
| Entry | Diene | Dienophile | Method | Adduct | Yield (%) |
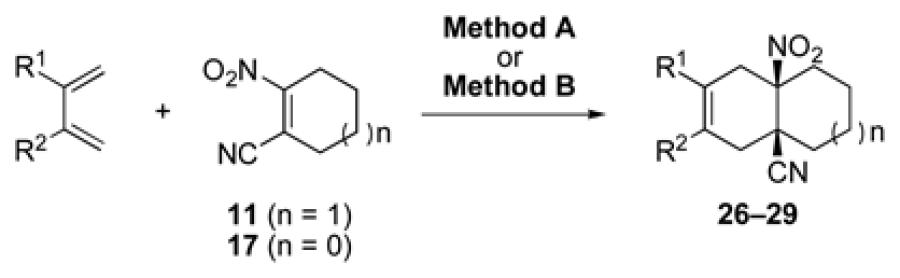
| 1 |
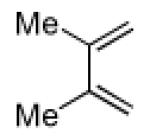
|
11 | A |
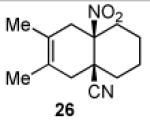
|
26 |
| 2 |
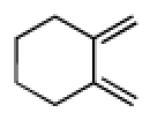
|
11 | A |
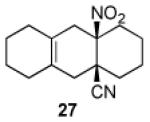
|
35 |
| 3 |
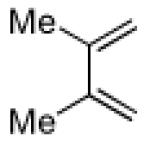
|
17 | B |
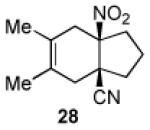
|
95 |
| 4 |
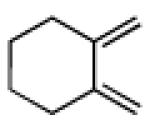
|
17 | B |
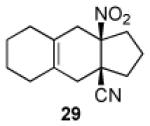
|
91 |
Key: Method A: 2,6-di-tert-butyl-4-methylphenol, 5.0 M LiClO4 in THF, 100 °C, 60 h; Method B: 2,6-di-tert-butyl-4-methylphenol, toluene, 100 °C, 24 h.
