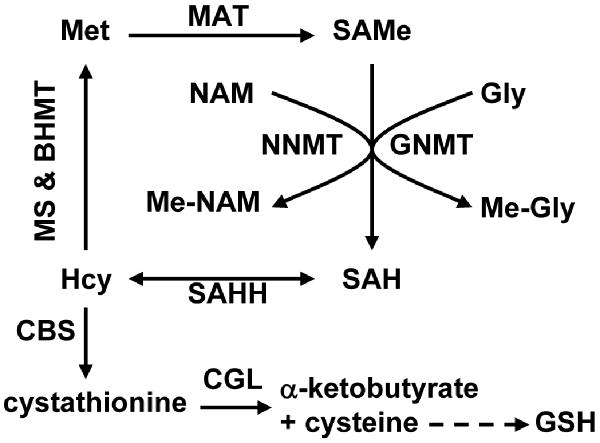
Figure 1.
Schematic representation of hepatic methionine metabolism. The first steps in hepatic methionine (Met) metabolism are conversion to S-adenosylmethionine (SAMe), through a reaction catalyzed by methionine adenosyltransferase (MAT), and transfer of the methyl group of SAMe to numerous methyl acceptors with formation of S-adenosylhomocysteine (SAH). Although there are a large number of SAMe-dependent methyltransferases, the reaction that quantitatively contributes most to the transmethylation flux is the methylation of glycine by glycine N-methyltransferase (GNMT, a reaction activated by SAMe) to form N-methyl-glycine (Me-Gly) (1,2). Accordingly, the liver of GNMT-KO mice shows a marked increase in the concentration of SAMe and aberrant methylation reactions of DNA and proteins (6). Nicotinamide (NAM) administration may be used to reduce the elevated levels of hepatic SAMe in GNMT-KO mice. NAM is methylated to form N-methyl-NAM (Me-NAM) by the enzyme NAM N-methyltransferase (NNMT). SAH is subsequently hydrolyzed to homocysteine (Hcy) by SAH hydrolase (SAHH), which is an important metabolic hub. Hcy can be remethylated to regenerate Met, via either methionine synthase (MS, a pathway inhibited by SAMe) and betaine homocysteine methyltransferase (BHMT), or used for the synthesis of cysteine and α-ketobutyrate as result of its transsulfuration. The transsulfuration pathway involves two enzymes: cystathionine β–synthase (CBS, which is activated by SAMe) and cystathionine γ-lyase (CGL). Cysteine is then utilized for the synthesis of glutathione (GSH), while α-ketobutyrate penetrates the mitochondria where is further metabolized. This coordinated modulation by SAMe of the flux of Hcy through the remethylation and transsulfuration pathways maximizes the production of cysteine, and consequently of GSH, after a methionine load minimizing the regeneration of this amino acid.