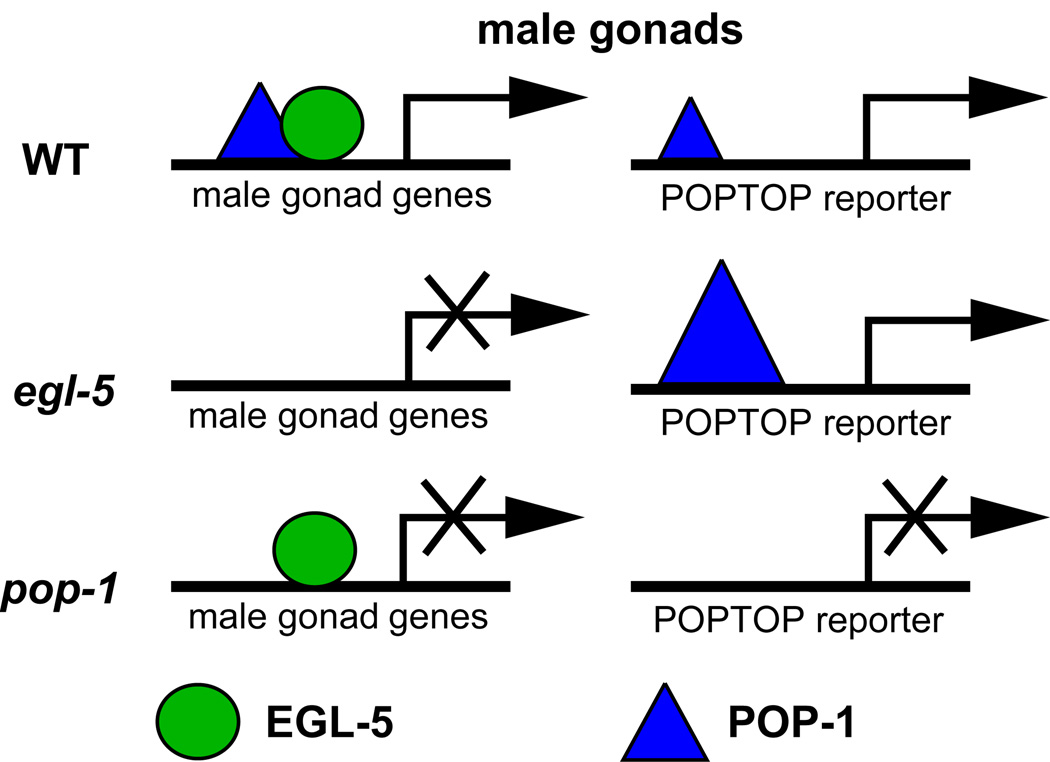
Fig. 6. Model for functional interaction of EGL-5 and POP-1 in the gonad.
Based on genetic and physical interaction, we suggest that EGL-5 helps recruit POP-1 to male gonadal target genes containing recognition sites for both proteins, imposing male-specific function on the gonadal Wnt/β-catenin pathway. In the absence of either EGL-5 or Wnt/β-catenin pathway members there is a failure to promote male specific cell fates because these shared targets are not activated. In this model the recruitment of POP-1 to male gonadal target genes by EGL-5 limits the amount of POP-1 available to activate the POPTOP reporter, accounting for the lower expression of this reporter in the male gonad. In egl-5 mutant males POP-1 is not recruited to male-specific target genes, allowing more efficient activation of the POPTOP reporter, which lacks EGL-5 recognition sites.