Abstract
Microinjections of D,L-homocysteic acid (DLH) were used to test the hypothesis that neuronal activation within the Botzinger complex area can modify the spatiotemporal characteristics of the cough reflex in 17 spontaneously breathing pentobarbitone anesthetized cats. DLH (50 mM, 1.25-1.75 nmol, 9 cats) reduced the number (P<0.01) of coughs and expiratory amplitude of abdominal electromyographic activity (P<0.01), and also esophageal pressure (P<0.001) during mechanically induced tracheobronchial cough. The duration of cough abdominal activity was shortened by 48% (P<0.05). DLH microinjections also temporarily reduced the respiratory rate (P<0.01) and increased the mean arterial blood pressure (P<0.001), baseline of esophageal pressure (P<0.01), and end tidal CO2 concentrations (P<0.01). Lower doses of DLH (0.27-0.35 nmol, 7 cats) or vehicle (25-35 nl, 8 cats) induced few alterations in cardiorespiratory or cough characteristics. The results support predominantly inhibitory effects of neurons in the region of the Bötzinger complex on cough abdominal activity and cough number.
Keywords: Bötzinger complex, cat, cough, DLH, ventral respiratory group
INTRODUCTION
The Botzinger complex (BOT) is a neuronal population of expiratory (E) neurons with an augmenting (aug) discharge pattern within the most rostral extension of the ventral respiratory column (VRC) in the vicinity of the retrofacial nucleus (1-3). The BOT region and surrounding reticular formation also contain some E units with decrementing (dec) discharge patterns (4) as well as other respiratory related neurons (5, 6). The BOT area and particularly the pre-Bötzinger complex (pre-BOT) are believed to be important in the generation of the respiratory rhythm and modulation on expiratory motor activity (2, 7). It has been also proposed that the respiratory network within the BOT/pre-BOT is a crucial part of the central pattern generation neuronal network for cough reflex (5, 6, 8).
The BOT neurons (E-aug and E-dec) have widely distributed axonal connections with other areas containing neurons with breathing-modulated activities. The majority of synaptic effects of BOT E units is inhibitory, and influence both I and E neurons throughout the VRC, the dorsal respiratory group, and phrenic motoneurons (1, 3). Studies utilizing a variety of methods and models of the respiration generating neuronal network suggest that BOT neurons participate in shaping and temporal control of I and E phases during breathing (see e.g. 2, 9). Neuronal assemblies containing BOT (BOT/pre-BOT) E-dec, E-aug early and E-aug late neurons are proposed to shape cough expiratory discharge as well (5, 8, 10). During cough, the activation of E-dec units promotes the termination of the cough I period. This activity also slightly postpones the activation of a subset of E-aug neurons in the BOT known as E-aug early, due to a temporal shift of their activity patterns during cough. E-aug early units are proposed to provide excitatory drive to premotor and motoneurons during cough expulsion (5, 8, 11, 12). Reciprocal inhibition of E-dec neurons by E-aug early neurons also is proposed to result in disinhibition of E-aug late neurons. E-aug late neurons participate in the termination of the active cough E phase because they suppress the discharge of E-aug early units, which drive cough expulsion. Selective (or unbalanced) excitation (or inhibition) of one of these neuronal populations would be expected to alter breathing as well as cough cycle timing. Based on Shannon's model (5, 8), excitation of E-dec and/or E-aug late neurons should result in suppression of cough expulsion as well.
Neurons in the region of the BOT were excited by a non-specific glutamate receptor agonist D,L-homocysteic acid (DLH) in earlier experiments reported by Bongianni et al (11, 13, 14) and McCrimmon et al (15). Microinjections of DLH led to the suppression of breathing expressed as a reduction of respiratory rate (RR) up to complete apnea and reduced amplitudes of I neuronal and nerve discharge. Higher doses of DLH also increased expiratory motor discharge (11, 13). Microinjections of DLH into the vicinity of BOT area also elicited other effects, such as alterations in blood pressure (14). In the present study we hypothesized that activation of BOT neurons would affect the spatiotemporal characteristics of the cough reflex, primarily by altering cough phase durations.
MATERIAL AND METHODS
Experiments were performed in 17 cats (4.3 ± 0.2 kg; 14 females and 3 males). The animals were anesthetized with sodium pentobarbital (35 mg/kg, i.v.) and supplementary doses were administered (1-3 mg/kg, i.v.) as needed. Atropine (0.1 mg/kg, i.v.) was given at the beginning of the experiment to reduce secretions. The trachea, femoral artery and vein were cannulated. The animals were allowed to spontaneously breathe a gas mixture of 40% oxygen, balance nitrogen. Arterial blood pressure (BP), end-tidal CO2 (ETCO2), and body temperature were continuously monitored. Body temperature was controlled by a heating pad and maintained at 37.5 ±0.5°C. Periodically samples of arterial blood were removed for blood gas and pH analysis. All procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals and approved by the University of Florida IACUC.
Electromyograms (EMG) of respiratory muscles were recorded with bipolar insulated fine wire electrodes. EMGs were recorded bilaterally from the expiratory transversus abdominis muscles (ABD) and inspiratory parasternal muscles (PS). The PS electrodes were placed at T3 proximal to the sternum after exposing the surface of the muscle. Transversus abdominis electrodes were placed 3-4 cm lateral to the linea alba. A soft balloon was inserted into the esophagus for a measurement of intrathoracic pressure changes (esophageal pressure – EP recording).
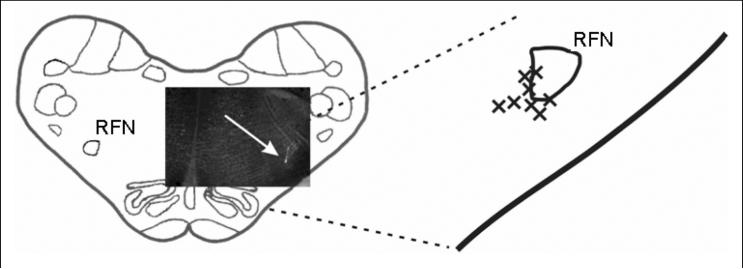
Animals were placed prone in a stereotaxic frame and the dorsal surface of medulla was exposed by an occipital craniotomy. The surface of the brainstem was covered by warm paraffin oil. Microinjection of the excitatory amino acid agonist DLH (10 or 50 mM, 25-35 nl) in artificial cerebrospinal fluid was used to excite neurons. Single-barrel (tip diameter 3-30 μm) or a composite 3-barrel glass micropipette with carbon fiber electrodes were used for pressure injection of solutions. The tip of the micropipette was positioned under stereotaxic control into the region of BOT (4.2-5.1 mm rostral to the obex, 3.0-3.5 mm lateral to the midline, 4.2-5.3 mm below the dorsal medullary surface). The injected volume was monitored by the observation of movement of the meniscus in the micropipette barrel with a microscope. Injection sites were labeled by fluorescent latex beads (16; Fig. 1). Moreover, the pipette tip was considered to be placed in the BOT region when multiunit E neuronal activity was recorded (9 out of 10 recording locations).
Fig. 1.
Diagrammatic reconstruction of DLH microinjection sites. The DLH solution was labeled by a fluorescent marker (bright dot pointed out by a white arrow) in the scheme of a transverse medullary section (4.5 mm rostral to the obex) with an inset photograph shown in the left hand side of the figure. The position of 8 reconstructed injection sites (out of 9 locations injected with 1.25 to 1.75 nmol of DLH) related to the retrofacial nucleus (RFN) is shown on the right hand side.
Tracheobronchial cough was induced by mechanical stimulation of the intrathoracic airways with a thin polyethylene catheter. This catheter was inserted into the trachea (and moved rostrocaudally and rotated) for periods of 10, 20, or 30 s to elicit repetitive coughing. Cough was defined by a large burst of I-related PS EMG activity immediately followed by a burst of E ABD EMG activity corresponding to the related I-E wave of EP.
All EMGs were amplified, filtered (300-5000 Hz), rectified, and integrated (time constant 200 ms). The number of coughs in response to mechanical stimulation of the trachea (average number of coughs per 10 s stimulation - CN), amplitudes of PS and ABD EMG moving averages and the peak I and E EP during appropriate phases of cough and breathing, RR, duration of inspiratory and expiratory phases (TI and TE), BP, and ETCO2 were analyzed. Monitored cardio-respiratory parameters were measured just before each cough trial. RR was calculated from 3-5 consecutive breathing cycles. Averages of these variables in each period (the control pre-microinjection, post-microinjection, and recovery) were calculated for statistical analysis. TI was defined as the period from the onset of PS EMG activity until its maximum during cough (or breathing). The cough TE was defined as the interval from the maximum of PS activity to the onset of the next parasternal EMG burst (17). In addition, we analyzed the duration of ABD EMG activity during the cough. The durations of the decrementing part of PS EMG activity (from its maximum to its offset, also known as postinspiratory activity) and the entire PS activation (TI plus postinspiratory duration) during quiet breathing vs. stimulation were compared as well.
Fifteen to 25 consecutive cough stimulation trials, separated by approximately 1 min, were conducted to establish a stable cough baseline. At this point in time, 3-4 control pre-injection trials were made with each trial separated by approximately 1 min. Another 3-4 trials were performed in the period 0-5 min after the microinjection followed by an additional 3-4 trials in the 8-15 min post-DLH microinjection interval. Magnitudes of the moving averages during coughing were normalized relative to the mean intensities of control pre-injection coughs. All cough parameters were averaged over each group of 3-4 trials.
After the experiment, the medulla was removed for histological processing. The tissue was fixed in 4% paraformaldehyde, followed by 30% sucrose solution. The frozen medulla was then cut into transverse slices (50 or 100 μm thick) by a freezing microtome. Sections were examined under UV (and light) microscopy for detection and localization of injection sites. We reconstructed the position of 16 out of the 17 injection locations (an example of 8 recognized out of the 9 locations where 1.25-1.75 nmol of DLH was microinjected is in Fig. 1) placed medially and ventrally to the retrofacial nucleus where clusters of BOT neurons are reported to be located (1-4, 11-13).
Results are expressed as mean values ±SE. For statistical analysis repeated measures ANOVA with Student-Newman-Kuels post tests and a paired t-test were applied as appropriate. The Pearson correlation analysis was performed for some variables. The differences of variables were considered significant if P<0.05.
RESULTS
Microinjections of DLH (8 cats, 1.39 ± 0.05 nmol, range 1.25-1.75 nmol, 9 injections, 8 locations; Fig. 1) into the BOT region suppressed tracheobronchial cough induced by mechanical stimulation (CN = 0.4 ± 0.2 compared to 4.0 ± 0.8 in control trials, P<0.01; Fig. 2). Cough was completely eliminated in 4 cats, residual cough reflexes were detected in another 4 animals. Cough recovered in the 8-15 min period after the microinjection (CN = 3.0 ± 0.8, P>0.05 vs. control, P<0.01 vs. post-DLH microinjection state). All the other parameters of coughs after recovery were not significantly altered compared with the control coughs (P>0.05) either.
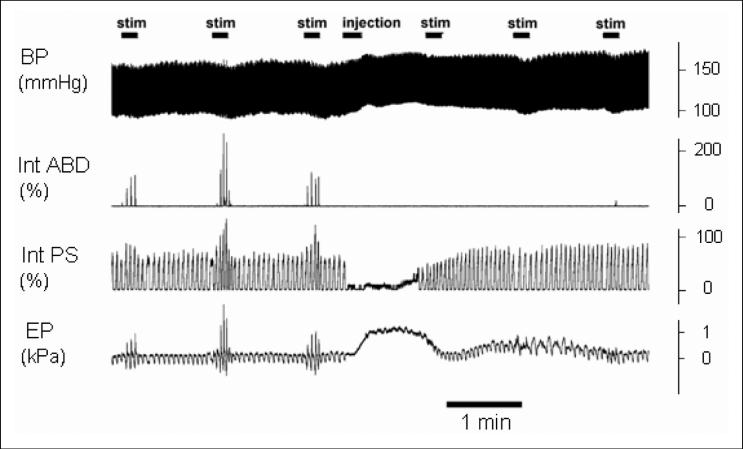
Fig. 2.
Effects of microinjection of DLH into the Bötzinger complex area (injection) on cough. The microinjection induced transient increases in arterial blood pressure (BP) and the esophageal pressure (EP), and a reduction in respiratory rate (apnea) and the amplitudes of parasternal EMG moving averages (Int PS). Cough was markedly suppressed due to the DLH microinjection (10 s stimulations - stim on the right hand side) compared with the control pre-injection coughs (stimulations on the left hand side), presented as a marked reduction or absence of expiratory abdominal EMG activity (Int ABD). The activations of the contralateral (left) parasternal and abdominal muscle EMGs are illustrated. The ipsilateral muscles were affected by the microinjection in a similar manner.
DLH microinjections also induced (Fig. 2) a transient decrease in RR (4.4 ± 2.1 vs. 21.4 ± 2.0 in the control; P<0.01; up to complete apnea in 4 cats, cough was eliminated in 3 of these 4 animals) and in peak PS EMG activity during breathing (31 ± 13% of the pre-injection level; P<0.01), and increases in BP (188 ± 10 from 129 ± 5 mmHg; P<0.001), EP (0.60 ± 0.15 from 0.10 ± 0.01 kPa, P<0.05), and in ETCO2 (46.8 ± 2.7 from 36.4 ± 1.6 mmHg; P<0.01). These alterations reached their maxima within 10-60 s after the beginning of microinjection and lasted from 30 s (the shortest RR reduction) up to 300 s (the longest increase in BP).
In a subset of animals (n=4), cardiorespiratory changes were less pronounced (and also shorter) and residual coughing was present after the DLH microinjections. These 4 animals, along with another cat (1.35 nmol of DLH microinjected into the BOT area), where the BP increase was prevented by i.v. administration of nitroprusside (0.05 mg/kg), were used for an analysis of cough parameters in the post-DLH microinjection period (5 cats, 1.36 ±0.04 nmol, range 1.25-1.5 nmol, 6 injections, 5 locations; Table 1). Along with the reduction of CN, reduced expiratory amplitudes of ABD EMG and EP were observed (Table 1). The cough TI and TE were not affected by the microinjection of DLH; however, the duration of cough ABD activity was shorter by 48% compared to pre-injection control (P<0.05; Table 1). Cardiorespiratory values in these 5 cats taken within the periods of stimulations (pre-microinjection, post-DLH microinjection, and the recovery intervals) showed non-significant variations of RR, BP, EP, and ETCO2. RR was slightly increased in the recovery period (Table 1).
Table 1.
Cough and cardiorespiratory parameters during control (pre-microinjection), DLH (0-5 min post-microinjection), and recovery (8-15 min post-microinjection) periods in 5 animals with cough responses that were not completely eliminated by DLH.
| Control | DLH | Recovery | |
|---|---|---|---|
| CN | 2.9 ±0.5 | 1.1 ±0.3* | 3.7 ±1.0+ |
| PS ips (%) | 94 ±11 | 71 ±16 | 107 ±15+ |
| PS con (%) | 94 ±12 | 67 ±16 | 107 ±15+ |
| ABD ips (%) | 95 ±18 | 42 ±13** | 98 ±22+ |
| ABD con (%) | 93 ±14 | 42 ±9** | 99 ±21++ |
| EP-I (kPa) | 0.36 ±0.04 | 0.33 ±0.05 | 0.38 ±0.04+ |
| EP-E (kPa) | 0.38 ±0.04 | 0.17 ±0.02*** | 0.40 ±0.10++ |
| cough TI (ms) | 1580 ±220 | 1510 ±190 | 1350 ±220 |
| cough TE (ms) | 2730 ±520 | 2520 ±450 | 2170 ±570 |
| T ABD (ms) | 860 ±220 | 450 ±150* | 820 ±260+ |
| RR (/min ) | 16.8 ±2.3 | 16.5 ±1.7 | 20.8 ±1.7***++ |
| BP (mmHg) | 119 ±5 | 130 ±10 | 123 ±5 |
| ETCO2 (mmHg) | 38.6 ±1.9 | 41.4 ±2.9 | 37.2 ±1.6 |
| breathing EP (kPa) | 0.13 ±0.05 | 0.11 ±0.02 | 0.08 ±0.02 |
CN, number of coughs per 10 s stimulation; PS ips, PS con, ipsilateral and contralateral parasternal muscle EMG moving average amplitudes; ABD ips, ABD con, ipsilateral and contralateral abdominal muscles EMG moving average amplitudes; EP-I, EP-E, inspiratory and expiratory amplitudes of esophageal pressure; cough TI, cough TE, the duration of inspiratory and expiratory cough phases; T ABD, the duration of abdominal muscle activation during cough; RR, respiratory rate; BP, mean arterial blood pressure; ETCO2, end-tidal concentration of CO2; breathing EP, baseline (quiet expiratory) esophageal pressure
P<0.05
P<0.01
P<0.001 compared with control
P<0.05
P<0.01 compared with DLH period.
Linear regression analysis was performed for the differences in RR, CN, BP, and cough ABD amplitudes (all 9 afore-mentioned cats were included). The only correlation significantly different from zero was found between CN and cough ABD amplitudes (P=0.014, r2=0.60).
Large inspiratory efforts still occurred during mechanical stimulation of the tracheobronchial airway after microinjection of DLH, even though the magnitude of the expulsive component of cough was depressed or eliminated. We examined the characteristics of these “residual” inspiratory efforts as well as inspiratory motor drive during breathing before and after microinjection of DLH. Peak amplitudes of PS EMGs during the tracheobronchial stimulation (including the “residual” cough inspirations) were reduced compared with the control cough inspirations (Table 2) by microinjection of DLH into the BOT area. However, there was no significant effect on the magnitude of EP during these inspirations (Table 2). The durations of inspiratory or postinspiratory phases of PS EMG (cough and “breathing” cycles) during tracheobronchial stimulation were not significantly altered compared to control respiratory cycles (Table 2) under both pre-microinjection and post-DLH microinjection conditions.
Table 2.
Spatiotemporal characteristics of inspiration during eupnea (resp) and during tracheobronchial stimulation (stim) before (control) and after the microinjection of DLH (DLH) into the Bötzinger complex area.
| Control resp | Control stim | DLH resp | DLH stim | |
|---|---|---|---|---|
| PS ips (%) | 35 ±13 | 93 ±12*** | 37 ±14 | 59 ±13++ |
| PS con (%) | 34 ±13 | 93 ±11** | 35 ±14 | 57 ±13++ |
| EP-I (kPa) | 0.15 ±0.02 | 0.36 ±0.05** | 0.17 ±0.03 | 0.30 ±0.06* |
| TI (ms) | 1090 ±110 | 1590 ±220 | 1300 ±190 | 1530 ±150 |
| T PS dec (ms) | 490 ±70 | 350 ±40 | 470 ±70 | 420 ±40 |
| T PS (ms) | 1590 ±140 | 1930 ±210 | 1770 ±230 | 1950 ±170 |
| T (ms) | 3550 ±300 | 4280 ±690 | 3830 ±420 | 4330 ±550 |
PS ips, PS con, ipsilateral and contralateral parasternal muscle EMG moving average amplitudes; EP-I, inspiratory amplitude of esophageal pressure; TI, inspiratory duration from the beginning of discharge to maximum activity; T PS dec, the duration of decrementing part of parasternal muscle activity; T PS, the duration of activation of parasternal muscles; T, total cycle duration
P<0.05
P<0.01
P<0.001 between respiration and stimulation
P<0.01 between control and DLH periods.
In 7 cats, lower doses of DLH (0.31 ± 0.01 nmol, range 0.27 - 0.35 nmol, 9 injections, 7 locations) were microinjected into the area of BOT. The responses to these DLH doses of RR, BP, EP, and the respiratory PS EMGs were inconsistent and not significantly affected by these microinjections. However, significant increases in peak ETCO2 (39.1 ± 2.0 from 34.7 ± 1.7 mmHg; P<0.05) were detected following these DLH microinjections. The number of coughs (3.3 ± 1.1 from 4.5 ± 1.1; P>0.05) and the amplitudes of ABD EMG and E EP during cough were not significantly different from control values (P>0.05).
Microinjections of artificial cerebrospinal fluid into the BOT area in 8 cats (34.1 ± 1.2 nl, 11 injections, 8 locations) had no significant effect on cardiorespiratory or cough parameters.
DISCUSSION
The major finding of this study is that the cough reflex was suppressed by excitation of neurons in the region of BOT with microinjection of the excitatory amino acid agonist DLH. This cough suppression was manifested as a reduction in cough number and in ABD EMG and E EP amplitudes. The duration of ABD activation was also markedly shortened due to the intervention.
We performed microinjections of the non-specific glutamate receptor agonist DLH into the area of BOT. The sites of our microinjections (see section Methods) were found in the medial and ventral vicinity of retrofacial nucleus and these locations were also characterized by the presence of multiunit E-aug discharge typical for BOT (1-3, 11-13). The BOT contains mainly E neurons with inhibitory connections to the majority of other respiratory neuronal populations within the medulla (1-3, 6). Excitation of these neurons by DLH caused suppression of respiration (11,13-15). Our microinjections of 1.25 to 1.75 nmol of DLH also induced such transient alterations in respiration. We observed some increases in BP, EP, and ETCO2 due to the microinjections. Bongianni et al (14) reported BP alterations in some of their microinjection sites (mostly ventral to retrofacial nucleus) within the rostral ventrolateral medulla, the area that overlaps the BOT region. We saw different levels of RR, BP, EP, and ETCO2 alterations in our experiments. There were few relationships among the changes of these variables (e.g., some pronounced decreases in RR were associated with small increases in BP such as that shown in Fig. 2). The variable alterations of EP and BP might be explained by different amounts of DLH diffusion to the neighboring areas of the rostral ambigual nucleus where esophageal motoneurons are located (18) and to the subretrofacial nucleus, which is an area that is linked with regulation of blood pressure (19). Increased ETCO2 is a consequence of respiratory suppression and it was a likely contributory component to the recovery of respiration after the microinjections (see RR later in post-DLH periods; Table 1). The effects of coughing on respiratory, cardiovascular, autonomic nervous, and other systems have been reported before (7, 20). It has been found that very high levels of hypercapnia are necessary to affect the cough reflex (21). On the other hand, little is known about the modulation of the cough by EP or BP. However, in a group of our 5 animals, in which detailed analysis of the cough motor pattern in the post-DLH microinjection period was performed, RR, EP, BP, and ETCO2 were not changed significantly (Table 1). This observation suggests that the suppression of cough was due to neuronal excitation within the BOT area, and not due to cardiorespiratory changes caused by the DLH microinjection.
Our current conceptualization of the neurogenesis of cough holds that there is a common respiratory/cough generating neuronal network in the brainstem (5, 6), but that there are also functional elements that control cough, which do not participate in the regulation of breathing (10). One such element, the cough gate, provides central excitatory input to the cough generating network, enables reconfiguration of the respiratory/cough pattern generator (R/CPG), and provides excitatory drive to expiratory premotor output during coughing (17, 22). Recently we have also unraveled a cough suppressive population of neurons located within the area of the caudal VRC and termed this population a cough suppressor (16). According to this concept, cough can be suppressed by inhibition of afferent inputs, by suppression of the excitatory gating mechanism, by activation of central cough suppressive mechanisms, or by a direct effect on R/CPG. Generalized inhibition of neurons in the R/CPG might result in alterations of most cough components such as a reduction of I and E activities and some changes in the temporal characteristics of cough. We observed, in the present study, a reduced CN, reduced E cough amplitudes, and shorter E ABD activation during coughing. Microinjection of DLH into the area of the BOT also reduced I efforts during tracheobronchial stimulations in post-DLH microinjection period (compared to pre-microinjection controls).
It is unlikely that microinjection of DLH into the BOT area altered the processing of cough-related afferent inputs. However, reduced activity of pulmonary stretch receptor caused by lower I efforts could attenuate coughing. Alterations in afferent signals (like that produced by unilateral vagotomy; 23) can affect cough phase durations, but that result was not observed in our study. Moreover, it has been proposed that although the presence of phasic pulmonary stretch receptor activity is necessary for tracheo-bronchial coughing to occur (7, 10, 23) it has very little influence on the cough E efforts (24). Coughing in paralyzed animals (5, 6) and a little correlation between I and E cough amplitudes in non-paralyzed animals (24) support this view.
The fact that I efforts during cough and the duration of ABD activity in post-DLH residual coughs were reduced by microinjection of DLH into the region of the BOT is not consistent with our previous findings regarding suppression of the cough gate or excitation of the cough suppressor (10, 16, 17, 22). However, our present data are consistent with the fact that the BOT area contains neuronal populations that are part of the R/CPG, that these neuronal populations are primarily inhibitory to other elements of the R/CPG, and that our microinjections affected the cough pattern generating network. It is unlikely that our microinjections induced a selective excitation of a homogenous population of neurons. Surrounding groups of neurons could be affected by the microinjection of DLH as well (see previous discussion of EP and BP effects, and for further discussion of the selectivity and specificity of the microinjection method see 14, 16, 25). Moreover, our intervention was placed unilaterally leaving the contralateral BOT area unaffected directly by DLH. The extent to which the activity of neurons in the contralateral BOT might have been altered secondarily to the microinjection of DLH (by synaptic effects from neurons near to the injection site) is unknown.
Inhibitory effects of microinjection of DLH within the BOT region on I components of induced responses were observed. These inspiratory efforts included those from “residual” coughs and other inspirations within the stimulation periods. We do not interpret these inspiratory bursts as eupneic inspiration, because almost all of them were enhanced by mechanical stimulation of the trachea. This observation is consistent with the inhibitory influence of BOT neurons to most of I neuronal populations in the medulla and the excitatory effects of cough-related stimulation. Following the DLH-induced period of apnea or reduced RR, the durations of I efforts were not significantly altered. This stability of TI is in line with the model of the cough generating network and other data (5, 8) showing that the cough TI is also determined by I-driver (I-plateau) neurons and by neuronal populations within the pontine respiratory group, which were not directly affected by our microinjections. Our results are consistent with a dominance of the synaptic effects of inhibitory E-dec and E-aug late neurons (both inhibitory to other E neurons in the medulla) within the areas in which DLH was microinjected. These neurons, when excited, could reduce the activation of E-aug early (E excitatory) neurons (and consequently E motor output) and shorten this activation without necessarily affecting the duration of the whole E phase and cycling during cough. This pattern of responses was observed in our experiments. Our data are not consistent with the report that the majority of neurons in the BOT region provide the excitatory drive to caudal VRC neurons in cough (12). It is possible that other cough specific mechanisms, which are similar to the cough suppressant circuits proposed by us for the caudal VRC (16), are involved in the control of cough within this area. However, several recording studies have not shown a high number of recruited units or non-respiratory modulated neurons that were modulated during cough within the region (5, 6, 12). These types of neurons could provide a neuronal substrate for a cough suppressive element in the regulatory system for this behavior (10). There may be excitatory drive to pre-motor and motor output during cough (and/or their disinhibition) from other brainstem structures related to the cough gating mechanism (17, 22). BOT neurons are likely to participate mostly in shaping the cough E activity. We did not see excitation of E motor output (either during breathing or during coughing), as was reported by Bongianni et al (11, 13). This may be because we employed lower doses of DLH in our study as compared with those (1.6–16 nmol) in the experiments of Bongianni et al (11, 13). Moreover, the animals in their experiments were vagotomized and mostly artificially ventilated, which is markedly different from our preparation.
The cough suppression lasted only up to 5 min after the microinjections. It is important to note that residual coughs were observed mainly during the later stimulations within post-microinjection period. This cough suppression and the sustainment of effects regarding the other recorded parameters correspond well to neuronal excitation induced by DLH (our own neuronal recordings; 25). However, RR changes were shorter in duration presumably because of increased drive from central chemoreceptors (due to increased PCO2 and ETCO2), which contributed to earlier recovery of breathing. Moreover, slightly but significantly increased RR was recorded in the recovery cough period (Table 1). We presume that a higher CO2 drive and a reduction in the inhibitory effects from BOT neurons could account for this observation.
Acknowledgements
This study was supported by NIH HL 70125.
Footnotes
Conflicts of interest: No conflicts of interest were declared in relation to this work.
REFERENCES
- 1.Merrill EG, Lipski J, Kubin L, Fedorko L. Origin of the expiratory inhibition of the nucleus tractus solitarius inspiratory neurones. Brain Res. 1983;263:43–50. doi: 10.1016/0006-8993(83)91198-8. [DOI] [PubMed] [Google Scholar]
- 2.Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog Neurobiol. 1990;35:429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- 3.Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Bötzinger complex in the cat. Exp Brain Res. 1990;81:639–648. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- 4.Manabe M, Ezure K. Decrementing expiratory neurons of the Bötzinger complex. I. Response to lung inflation and axonal projection. Exp Brain Res. 1988;72:150–158. doi: 10.1007/BF00248510. [DOI] [PubMed] [Google Scholar]
- 5.Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol. 1998;84:2020–2035. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- 6.Shannon R, Baekey DM, Morris KF, Li Z, Lindsey BG. Functional connectivity among ventrolateral medullary respiratory neurones and responses during fictive cough in the cat. J Physiol. 2000;525:207–224. doi: 10.1111/j.1469-7793.2000.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jakus J, Tomori Z, Stránsky A. Neuronal Determinants of Breathing, Coughing and Related Motor Behaviours: Basics of Nervous Control and Reflex Mechanisms. Martin; Wist: 2004. [Google Scholar]
- 8.Shannon R, Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG. Production of reflex cough by brainstem respiratory networks. Pulm Pharmacol Ther. 2004;17:369–376. doi: 10.1016/j.pupt.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Balis UJ, Morris KF, Koleski J, Lindsey BG. Simulations of a ventrolateral medullary neural network for respiratory rhythmogenesis inferred from spike train cross-correlation. Biol Cybern. 1994;70:311–327. doi: 10.1007/BF00200329. [DOI] [PubMed] [Google Scholar]
- 10.Bolser DC, Poliacek I, Jakus J, Fuller DD, Davenport PW. Neurogenesis of cough, other airway defensive behaviors and breathing: a holarchical system? Respir Physiol Neurobiol. 2006;152:255–265. doi: 10.1016/j.resp.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bongianni F, Corda M, Fontana G, Pantaleo T. Expiration-related neurons in the caudal ventral respiratory group of the cat: influences of the activation of Bötzinger complex neurons. Brain Res. 1990;526:299–302. doi: 10.1016/0006-8993(90)91235-9. [DOI] [PubMed] [Google Scholar]
- 12.Bongianni F, Mutolo D, Fontana GA, Pantaleo T. Discharge patterns of Bötzinger complex neurons during cough in the cat. Am J Physiol. 1998;274:R1015–R1024. doi: 10.1152/ajpregu.1998.274.4.R1015. [DOI] [PubMed] [Google Scholar]
- 13.Bongianni F, Fontana G, Pantaleo T. Effects of electrical and chemical stimulation of the Bötzinger complex on respiratory activity in the cat. Brain Res. 1988;445:254–261. doi: 10.1016/0006-8993(88)91187-0. [DOI] [PubMed] [Google Scholar]
- 14.Bongianni F, Corda M, Fontana GA, Pantaleo T. Excitatory and depressant respiratory responses to chemical stimulation of the rostral ventrolateral medulla in the cat. Acta Physiol Scand. 1993;148:315–325. doi: 10.1111/j.1748-1716.1993.tb09562.x. [DOI] [PubMed] [Google Scholar]
- 15.McCrimmon DR, Monnier A, Hayashi F, Zuperku EJ. Pattern formation and rhythm generation in the ventral respiratory group. Clin Exp Pharmacol Physiol. 2000;27:126–131. doi: 10.1046/j.1440-1681.2000.03193.x. [DOI] [PubMed] [Google Scholar]
- 16.Poliacek I, Corrie LW, Wang C, Rose MJ, Bolser DC. Microinjection of DLH into the region of the caudal ventral respiratory column in the cat: evidence for an endogenous cough-suppressant mechanism. J Appl Physiol. 2007;102:1014–1021. doi: 10.1152/japplphysiol.00616.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. J Appl Physiol. 1999;86:1017–1024. doi: 10.1152/jappl.1999.86.3.1017. [DOI] [PubMed] [Google Scholar]
- 18.Lawn AM. The localization, by means of electrical stimulation, of the origin and path in the medulla oblongata of the motor nerve fibres of the rabbit oesophagus. J Physiol. 1964;174:232–244. doi: 10.1113/jphysiol.1964.sp007484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dampney RA. The subretrofacial vasomotor nucleus: anatomical, chemical and pharmacological properties and role in cardiovascular regulation. Prog Neurobiol. 1994;42:197–227. doi: 10.1016/0301-0082(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 20.Korpas J, Tomori Z. Cough and other respiratory reflexes. S Karger, Basel; New York: 1979. [Google Scholar]
- 21.Tatár M, Korpas J, Polacek H, Zahradny V. Changes induced by severe hypoxia in respiratory defence reflexes in anaesthetized cats. Respiration. 1986;49:114–121. doi: 10.1159/000194868. [DOI] [PubMed] [Google Scholar]
- 22.Bolser DC, Davenport PW. Functional organization of the central cough generation mechanism. Pulm Pharmacol Ther. 2002;15:221–225. doi: 10.1006/pupt.2002.0361. [DOI] [PubMed] [Google Scholar]
- 23.Hanacek J, Davies A, Widdicombe JG. Influence of lung stretch receptors on the cough reflex in rabbits. Respiration. 1984;45:161–168. doi: 10.1159/000194614. [DOI] [PubMed] [Google Scholar]
- 24.Hanacek J, Tatar M, Widdicombe J. Regulation of cough by secondary sensory inputs. Respir Physiol Neurobiol. 2006;152:282–297. doi: 10.1016/j.resp.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Lipski J, Bellingham MC, West MJ, Pilowsky P. Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. J Neurosci Methods. 1988;26:169–179. doi: 10.1016/0165-0270(88)90166-5. [DOI] [PubMed] [Google Scholar]