Table 4.
One-Pot Synthesis of Benzofuransa
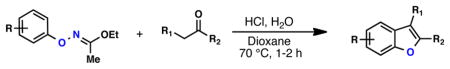 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Ketone | Product | Yield [%] | Time [h] |
| 1 | 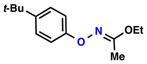 |
86% | 1 | ||
| 2 | 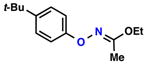 |
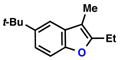 |
68% | 1 | |
| 3 | 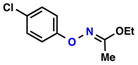 |
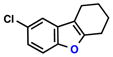 |
88% | 1 | |
| 4 | 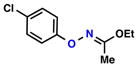 |
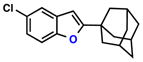 |
83% | 2 | |
| 5 | 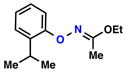 |
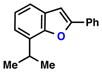 |
68% | 2 | |
| 6 | 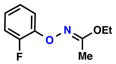 |
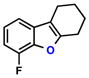 |
55% | 2 | |
Oxime (1 equiv), ketone (2 equiv), H2O (5 equiv), HCl (4 M in dioxane, 5 equiv), 1,4-dioxane (0.2 M), 70 °C, 1–2 h; isolated yield, average of 2 runs on a 0.5 – 1.0 mmol scale.
