Figure 4. PNPASE Augments RNase P, 5S rRNA, and MRP RNA Import into Yeast Mitochondria.
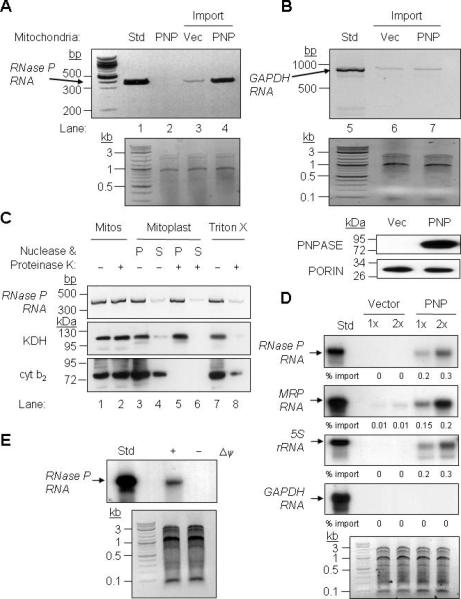
(A) Upper- In vitro transcribed human RNase P RNA was incubated with yeast mitochondria expressing human PNPT1 (PNP) or an empty vector (Vec) control. Non-imported RNA was digested with nuclease and the imported RNA was detected by RT-PCR. PNPT1-expressing mitochondria without added RNase P RNA was included as a specificity control for import and RT-PCR (lane 2 – Std, 1% of the reaction). Lower- Control showing equivalent total mitochondrial nucleic acid in each reaction.
(B) Upper- As in panel A, with cytosolic human GAPDH RNA used as a substrate. Middle- Control showing equivalent total mitochondrial nucleic acid in each reaction. Lower- Western blot showing PNPASE expression and PORIN immunoblot showing equivalent mitochondria in each import assay.
(C) After import as in panel A, mitochondria were subjected to osmotic shock, fractionated by centrifugation into soluble (S) and pellet (P) fractions, followed by proteinase K and nuclease additions where indicated. The pellet fraction was solubilized with Triton X-100 to expose the matrix. Localization was determined by RT-PCR for RNase P RNA and immunoblot for KDH (matrix) and cyt b2 (IMS) proteins.
(D) Upper- Radiolabeled RNase P, MRP, 5S rRNA, and GAPDH human RNAs were in vitro transcribed and then incubated with yeast mitochondria expressing PNPASE or an empty vector control. Non-imported RNA was digested with nuclease, followed by RNA isolation, separation on a urea acrylamide gel, and autoradiography. Import reactions were repeated with 1X and 2X amounts of RNA. Lower- Control showing equivalent total mitochondrial nucleic acid in each reaction.
(E) Upper- As in panel A except that the mitochondrial membrane potential (Δψ) was dissipated prior to import. Lower- Control showing equivalent total mitochondrial nucleic acid in each reaction.
