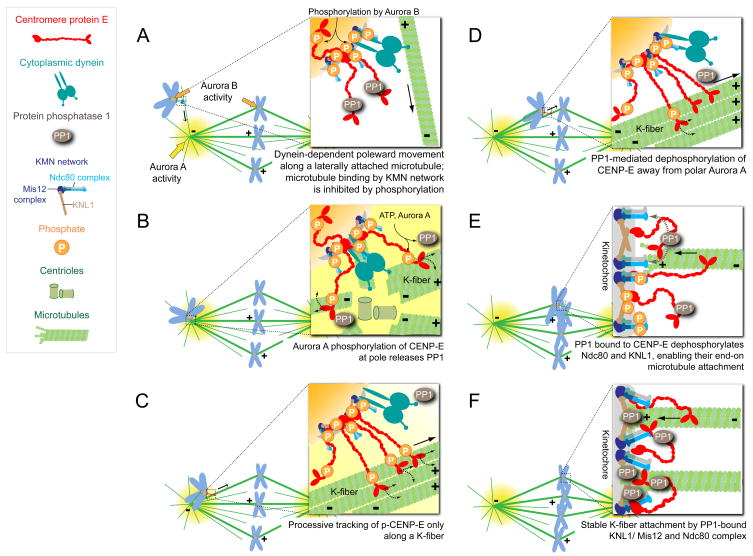
Figure 7.
A model of CENP-E regulation by Aurora kinases and PP1. (A) Unattached chromosomes are translocated poleward along a single astral microtubule in a dynein-dependent manner (Rieder and Alexander, 1990). (B) Aurora A phosphorylates CENP-E at T422 near the spindle poles, releasing PP1 from CENP-E. Phosphorylation of CENP-E provides selectivity toward bundles of kinetochore microtubules (K-fiber) for CENP-E to glide along. (C) Our evidence here demonstrates that CENP-E powers the congression of polar chromosomes to the spindle equator along the K-fiber of an already bioriented chromosome, as earlier proposed (Kapoor et al., 2006). (D) As chromosomes congress, kinetochores move away from the Aurora A gradient concentrated at the spindle poles and CENP-E is dephosphorylated. (E) Dephosphorylated CENP-E recruits a high local concentration of PP1 to the outer kinetochores of chromosomes it has translocated away from a pole. CENP-E delivered PP1 is essential for stable, microtubule capture by the kinetochores of these towed chromosomes through its dephosphorylation of key components at the kinetochore-microtubule interface (e.g. Ndc80 and KNL1), therby increasing their affinity for microtubules. (F) Upon biorientation, kinetochore substrates become separated from the inner centromeric Aurora B and kinetochore-recruited PP1 through its direct binding to KNL1 and CENP-E stabilizes kinetochore-microtubule interactions.