Abstract
Background
Surgical resection is the standard of care for patients with resectable non-small cell lung cancer or selected patients with pulmonary metastases. However, for high-risk patients radiofrequency ablation (RFA) may offer an alternative option. The objective of this study was to evaluate computed tomography guided (CT-guided) RFA for high-risk patients and report our initial experience in 100 consecutive patients by a thoracic surgical service.
Methods
Medically inoperable patients were offered RFA. Thoracic surgeons evaluated and performed RFA under CT guidance. Patients were followed in the thoracic surgery clinic. The primary endpoint evaluated was overall survival.
Results
One hundred patients underwent image-guided RFA for lung neoplasm (40 men, 60 women; median age 73.5 years; range 26-95). Forty-six (46%) patients with primary lung neoplasm, 25 (25%) with recurrent cancer and 29 (29%) with pulmonary metastases underwent RFA. The mean follow-up for alive patients was 17 months. The median overall survival for entire group of patients was 23 months. The probabilities of 2-year survival for the entire group, primary lung cancer patients, recurrent cancer patients and metastatic cancer patients were 49 % (CI 37- 60), 50 %(CI 33-65), 55% (CI 25- 77) and 41% (CI 19-62), respectively.
Conclusion
Our experience indicates that image-guided RFA done by the thoracic surgeons is feasible and safe in high-risk patients with lung neoplasm with reasonable results in patients who are not fit for surgery. Thoracic surgeons can perform RFA safely, and should continue to investigate this new image-guided modality which may offer an alternative option in medically inoperable patients.
Introduction
Surgical resection is the standard treatment in resectable disease and offers the best chance of cure particularly in the earlier stages (1). The standard treatment for early stage non-small cell lung carcinoma (NSCLC) is a lobectomy (2). However, a significant proportion of patients, particularly elderly patients with associated co-morbidities, are not candidates for surgery and these patients either receive no treatment or are treated with conventional external beam radiotherapy. In recent studies of patients with early stage NSCLC, who received no treatment, the median survival was 9-14 months (3,4). After treatment with external beam radiation for early stage NSCLC, 5-year survival rates are 10-30% (5-9). Sibley reviewed the results of conventional radiotherapy for Stage I NSCLC from Duke University in 156 patients and reported a median survival of 18 months (6). Thus, the results of conventional radiation therapy have not been satisfactory, prompting investigators to study other modalities, such as radiofrequency ablation (RFA) and stereotactic radiosurgery, for the treatment in this group of high-risk patients with lung cancer.
Surgical resection is also beneficial for selected patients with pulmonary metastases. Patients who have a single metastasis, prolonged disease-free survival, complete control of the primary tumor and no evidence of extra-thoracic disease are good candidates for resection (10). In medically inoperable or unresectable patients with pulmonary metastases, there are few effective options. These tumors are typically not sensitive to radiation or require a large field of radiation. Therefore, newer technologies, such as RFA, may also offer an alternative in the management of medically inoperable or high-risk patients with lung neoplasm.
RFA is administered via a thermal energy delivery system that applies an alternating current supplied by a radiofrequency energy generator and delivered through a needle electrode. The needle electrode is introduced percutaneously under computed tomography (CT) guidance and the tines are deployed within the tumor. This allows for maximal distribution of energy. The alternating current creates ionic agitation, generating heat that can reach 90°C. This leads to coagulative necrosis and tissue destruction in the area of the probe (11).
In this paper, we present our results with the use of image-guided RFA for the treatment of lung neoplasm in 100 consecutive patients by a thoracic surgical service. Our objective was to determine the outcomes of RFA in the treatment of lung neoplasm.
Methods
We retrospectively evaluated our experience with image-guided RFA for the treatment of lung neoplasm in medically inoperable patients at the University of Pittsburgh from 2000 to 2007. Informed consent for treatment with RFA was obtained from all patients. The study was approved by the Institutional Review Board at the University of Pittsburgh.
Selection of patients
Patients with primary lung neoplasm were routinely staged with chest CT scan and most patients (39/46; 84.8%) also underwent a positron emission tomography (PET) scan. Patients with primary lung cancer with mediastinal lymph nodes greater than 1 cm in short axis or a positive PET scan underwent mediastinoscopy. In patients with primary lung cancer, mediastinoscopy was performed in 9 patients and left video-assisted thoracoscopy was performed in 1 patient to biopsy hilar and aorto-pulmonary window nodes. The inclusion criteria for RFA in the treatment of patients for this study were (1) Patients who were considered medically inoperable due to a) poor pulmonary function (predicted post-operative FEV1 less than 40%, predicted post-operative DLCO <40%) (12) or b) high cardiac risk which includes severe coronary or valvular disease, and uncompensated congestive heart failure as described by the peri-operative guidelines for risk assessment in non-cardiac surgery by the American College of Cardiology/ American Heart Association (13) and/or other co-morbidities; (2) Patients who had failure of previous therapies or (3) Patients who refused surgical resection. Exclusion criteria included central tumors (within 3 cm of the hilum). All patients were evaluated by a thoracic surgeon to determine inoperability and suitability for RFA.
Treatment Protocol
Technique
A percutaneous CT-guided approach was used in all patients and thoracic surgeons performed all procedures as described previously (14,15). The majority of patients (n=86) underwent the procedure under general anesthesia. The electrosurgical needle's deployment was staged according to the size of the tumor and the manufacturer's suggested algorithm was followed. In one system (Boston Scientific, Natick, Massachusetts), an impedance-based algorithm was used and with another system (RITA Medical Systems, Inc., Moutainview, California) a temperature-based algorithm was used. With both these systems, the electrode was repositioned as many times as necessary in order to ablate the target tissue and a small rim of approximately 0.5-1cm of non-diseased pulmonary tissue to ensure adequate tumor margins.
Post-procedure follow-up
Follow-up of patients and Assessment of response
Patients were followed in four month intervals with clinical examinations, CT scans and, selectively, with PET scans. A modified RECIST criterion incorporating CT scan and PET scan was utilized to assess initial response to treatment (15). We evaluated initial response rate, time to local progression and overall survival.
Data Collection and Statistical Analysis
Information on patient demographics, tumor characteristics, treatment, and comorbidities (Charlson comorbidity index, CCI) was collected. CCI (16) was originally described to assess the impact of comorbidity on survival in hospitalized patients. In this index, a total of nineteen conditions, found to significantly influence survival, are assessed and a weighted score is given based on the relative risk. Specific endpoints studied were complications, clinical response rates, time to progression (TTP), and overall survival. All analyses were performed from the time of the first RFA session. The pretreatment CT scan was used as a baseline for evaluation of response and disease progression. Local disease progression of the treated nodule was assessed in accordance with the modified RECIST criteria in comparison with baseline diameter. TTP was calculated from the treatment date. Kaplan-Meier plots were constructed using Greenwood confidence limits. Log rank test was used to determine differences between groups. Association between categorical variables was tested with Fisher's exact test or the Chi square test.
Results
Patient Characteristics
One hundred patients underwent image-guided RFA over a 7 year period. There were 40 men and 60 women with a median age of 73.5 years (range 26-95). There were 46 (46/100; 46%) patients with primary lung neoplasm (all stages), 25 (25/100, 25%) with recurrent lung cancer and 29 (29/100, 29%) with metastatic disease. Patient characteristics are summarized in Table 1. A total of 109 lesions were treated with RFA in these 100 patients. In 92 patients, a single lesion was treated. In 8 patients, 2-3 lesions were treated in a single session. These patients had significant co-morbidities as indicated by a mean CCI score of 7.5 (median 7; range 2-13) (Table 2).
Table 1. Patient Characteristics.
| Gender: Male : Female 40:60 | |
| Age: | |
| Mean: | 72.8 |
| Median | 73.5 |
| range: | 26 – 95 |
| Histology | |
| Primary Lung Neoplasm (46) | |
| Squamous | 18 |
| Adenocarcinoma | 10 |
| NSCLC1, not specified | 17 |
| Others | 1 |
| Recurrent Lung Neoplasm (25) | |
| Squamous | 6 |
| Adenocarcinoma | 5 |
| NSCLC, not specified | 14 |
| Metastatic (29) | |
| Colorectal Cancer | 13 |
| Breast | 2 |
| Renal Cell | 3 |
| Sarcoma | 5 |
| Cervical | 2 |
| Squamous cell tongue | 1 |
| Testicular | 1 |
| Pheochromocytoma | 1 |
| Esophageal | 1 |
| Reason for RFA2 | |
| Poor PFT's3 | 43 |
| Increased Cardiac Risk | 18 |
| Failed Previous Therapy | 34 |
| Multiple comorbidities | 29 |
| Refused Surgery | 7 |
NSCLC: Non-small cell lung cancer
RFA: Radiofrequency ablation; some patients had more than one reason for RFA
PFT's: Pulmonary Function Tests
Table 2. Reason for RFA and comorbidities stratified by group*.
| 1° Lung ca. | Recurrent lung ca. | Metastatic lung ca | |
|---|---|---|---|
| Poor lung function | 26 | 13 | 4 |
| Increased cardiac risk | 12 | 3 | 3 |
| Failed other therapies | 1 | 14 | 19 |
| Multiple comorbidities | 18 | 4 | 7 |
| Refused Surgery | 3 | 1 | 3 |
| Charlson Comorbidity Index | |||
| 1° Lung ca. | Recurrent lung ca. | Metastatic lung ca | |
| CCI mean | 6.5 | 8.4 | 8.2 |
| CCI median | 6 | 9 | 8 |
Some patients had more than 1 reason for the use of RFA
RFA: Radiofrequency ablation; ca: cancer
The most common reason for RFA was poor pulmonary function tests precluding resection (Table 1, 2). The median FEV1 in these patients was 0.81 (44% of predicted) and median predicted DLCO was 35%. The reasons for RFA and the co-morbidity index stratified by group are shown in Table 2.
Peri-procedure course
The median hospital stay was 2 days (range 1-33).The most common complication was pneumothorax requiring a pigtail catheter in 59 (59%) patients. Prolonged air leak (>5 days) occurred in 7 patients (7%). Other complications included bleeding in 1 patient requiring bronchoscopy; myocardial infarction, cerebrovascular accident, deep vein thrombosis and respiratory failure in 1 patient, pleural effusions requiring drainage in 3 patients, and arrhythmias in 6 patients. There was one death within 30 days of the procedure which occurred after the patient was discharged (death occurred 2 weeks after the procedure).
Response to treatment
Initial response was determined by the modified RECIST criteria (14). The response could not be evaluated in 9 patients. In the remaining patients, an initial complete response was observed in 21% of patients, a partial response was observed in 41%. Stable disease was noted in 20% and progressive disease occurred in 18% of patients.
Time to Progression
During follow-up, local progression of the treated lesion occurred in 35 patients (35%) and the median time to local progression was 15 months (95% confidence interval 8-27 months). Overall progression (all sites) occurred in 60 patients and the median time to overall progression was 7 months (95% confidence interval 6-11 months). The median times to overall progression by type of neoplasm were: primary lung neoplasm (all stages), 7 months (95% confidence interval 6-15 months); recurrent neoplasm, 7 months (95% confidence interval 6-16 months); and metastatic lung neoplasm, 8 months (95% confidence interval 4-17 months).
Survival
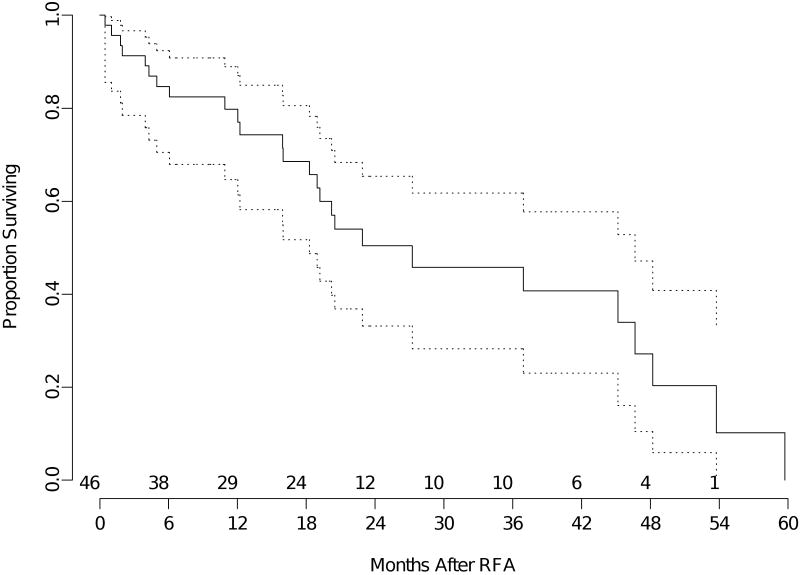
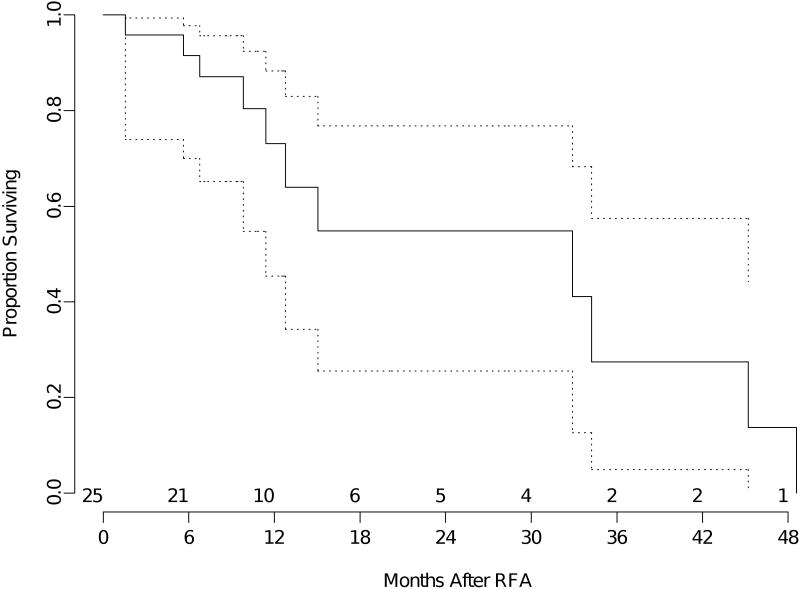
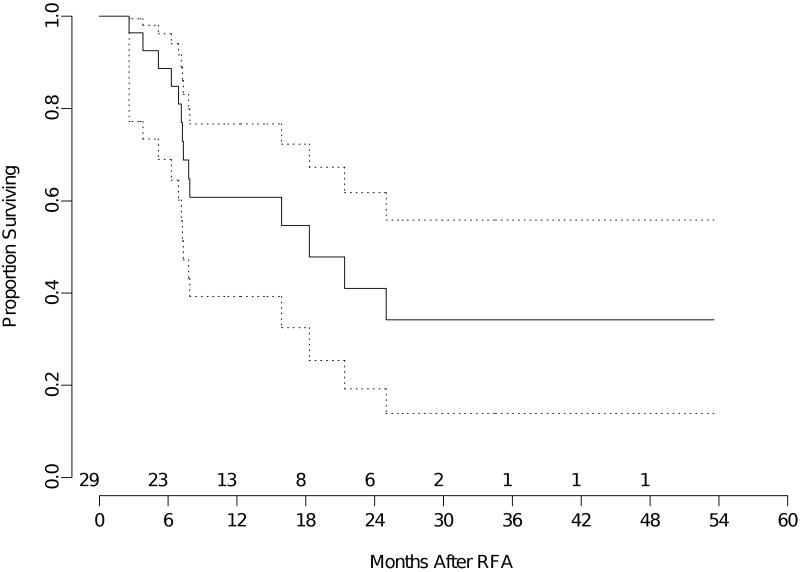
The median overall survival for the entire group of patients was 23 months (95% confidence interval 18- 37 months). The mean follow-up was 17 months (median 12 months). The median overall survival for patients with primary lung neoplasm (all stages) was 27 months (95% confidence interval 18-47 months) (Figure 1). Median overall survival was 33 months (95% confidence interval 11-45 months) for recurrent lung neoplasm (Figure 2) and 18 months (95% confidence interval 7 months -NR) for metastatic disease (Figure 3).
Figure 1.
Kaplan-Meier analysis of overall survival of patients with primary lung cancer (all stages). The time shown is in x axis is in months from RFA. The dotted lines are 95% confidence bands for the probability of overall survival. The number of patients at risk at the start of each 6 month interval is included above the x axis.
Figure 2.
Kaplan-Meier analysis of overall survival of patients with recurrent lung cancer. The time shown is in x axis is in months from RFA. The dotted lines are 95% confidence bands for the probability of overall survival. The number of patients at risk at the start of each 6 month interval is included above the x axis.
Figure 3.
Kaplan-Meier analysis of overall survival of patients with metastatic lung cancer. The time shown is in x axis is in months from RFA. The dotted lines are 95% confidence bands for the probability of overall survival. The number of patients at risk at the start of each 6 month interval is included above the x axis.
The estimated 2-year overall survival for the entire group was 49% (95% confidence interval 37-60%). The estimated 2-year overall survival for patients with primary lung neoplasm all stages) was 50% (95% confidence interval 33-65%). Estimated 2-year overall survival for patients with recurrent lung neoplasm was 55% (95% confidence interval 25-77%), and estimated 2-year overall survival for patients with metastatic disease was 41% (95% confidence interval 19-62%).
Comment
Surgical resection is the preferred treatment for patients with early stage lung cancer (1, 2) and surgical treatment is also beneficial in selected patients with metastatic and recurrent lung cancer (10,17). Unfortunately, patients who are medically inoperable but otherwise resectable have few effective options. In patients with Stage I NSCLC, who receive no treatment or who refuse treatment, a recent study showed that the estimated five year survival was 6% and the median survival was 9 months (3). In another study of 129 patients with early stage NSCLC, patients who did not receive any treatment had a median survival of 14 months, significantly lower than patients who were treated with surgery (46 months) or radiotherapy (19.9 months). In addition, the cause of death for patients who were not treated was related to cancer in 53% of patients, suggesting that treatment is beneficial in non-surgical patients (4). In the current study, the estimated median survival of 27 months after treatment with RFA compares favorably to either no treatment or standard external beam radiation.
In this high-risk patient population with lung cancer, newer modalities, such as RFA or stereotactic radiosurgery, may be applicable (15,18). This study indicates that image-guided RFA done by the thoracic surgeons is feasible and safe in high-risk patients with lung neoplasm with reasonable results in patients who are not fit for surgery. The assessment of response after RFA is difficult because, unlike surgical resection, there is a lesion or scar which remains after therapy. The reported response rates vary considerably in the literature with differing criteria being applied to assess response. We have adopted strict criteria and have utilized a modified RECIST criterion to evaluate response in these patients after RFA. However, this criterion has to be validated. (15)
Patients with recurrent lung cancer after surgical resection comprise a difficult group of patients. Importantly, treatment of recurrent disease is associated with a survival benefit. Sugimura and associates from the Mayo Clinic reported the results in 390 patients who developed recurrent cancer after complete surgical resection (17). The median post-recurrence survival in their study was 8.1 months, with estimated 1- and 2- year overall survivals of 37% and 17%, respectively. Surgical treatment for recurrences limited to the lung, was performed in a very selected group of patients and when the treatment included surgery, median survival was 32.8 months. Median survival was only 13.4 months for non-surgical treatment and 8.4 months for no treatment. In our study, RFA for the treatment of recurrent lung cancer resulted in a median survival of 33 months. These results appear reasonable, and in non-operative candidates, RFA may provide an alternate option.
Metastatic Lung Cancer
The International Registry for Lung Metastases reported the results in 5206 patients, who were treated with surgical resection for pulmonary metastases (10). The estimated 5-year survival after complete resection was 36% and the median survival was 35 months. Recurrences occurred in 53% of patients and the median time to recurrence was 10 months. When the metastases were resectable and when all factors were favorable, the median survival was 61 months; when they were unresectable, the median survival was 14 months with an estimated 5-year overall survival of 5%.
In the current study, the pulmonary metastases group was heterogenous. The median survival in this diverse group of patients was 18 months and the overall 2 year survival was 41%. Other investigators have also reported the results of RFA in the treatment of metastatic lung tumors (19-23). Yan and colleagues reported the results of RFA for the treatment of pulmonary metastases in 55 patients with primary colorectal neoplasms (19, 20). The median survival in these patients was 33 months and the estimated 2-year survival was 64%. While these results do not appear to be equivalent to complete surgical resection when possible, RFA may offer an alternative in a select group of high-risk patients, who are unable to undergo surgical therapy.
It is important to emphasize that the patients in our study had significant associated comorbidities, with a median Charlson score of 7. This index has been validated in a cohort of surgically resected patients with NSCLC in a study of 205 patients (24). The score was divided into four grades of increasing severity of the CCI index, with a CCI >5 representing the highest grade of comorbidities. For every increase in grade of CCI, there was an increase in the risk of adverse outcome after surgery. It is very important that a qualified thoracic surgeon evaluate the patient for assessment of resection and determination of medical inoperability, because of these complicating co-morbidities,. For example, in patients with upper lobe predominant emphysema and coexisting neoplasm, surgical resection may be feasible even if the pulmonary function tests appear borderline. Choong and colleagues reported a series of 21 clinical Stage I NSCLC with a mean FEV1 of 0.7 (29% of predicted) who underwent surgical resection with upper lobe predominant emphysema (25). In patients with pathological Stage I, overall survival was estimated to be 79% at 3 years.
One of the unique aspects of this series of 100 consecutive patients is that all the procedures were performed under CT-guidance by thoracic surgeons. Another large series from the United States was reported by Simon and colleagues, who evaluated the results in 153 patients (23). Their procedure-related mortality was 2.6% and the 30-day mortality was 3.9%. The mortality of 1% in our current series compares favorably with this report. The most common complication of RFA was a pneumothorax which was effectively treated with a pig tail catheter in most patients. Although serious complications were rare in our series, this group of patients had significant co-morbidities with a high co-morbidity index. It is, therefore, very critical that the procedure be performed by a team that follows these patients closely and also manages complications effectively. It is also important to follow these patients long term, similar to the follow-up for lung cancer patients after surgical resection. Thoracic surgeons are, therefore, ideally positioned not only to perform RFA but also provide peri-operative care and long-term follow-up for patient receiving RFA.
Decreasing Local Progression
The incidence of local progression was significant in this study (35%) as well as in studies by other investigators (21, 26). There are several factors which may influence local recurrence or progression of disease. The important technical issues include the degree of ablation, whether complete ablation is achieved and the adequacy of the margins of ablation around the tumor. Our data on margins and sublobar resection demonstrated that when the margins were less than 1 cm, the rate of local recurrence was 14% whereas local recurrence was 7% when the margins were greater than 1 cm (27). In general, we strive to attain a 0.5 to 1.0 cm margin around the tumor. In addition, in our current protocol, we limit RFA to lesions less than 5 cm and limit RFA to 3 or fewer lesions in a single session. Experimental studies have also demonstrated increases in the area of ablation by increasing conductivity with saline infusion (28). Hiraki and colleagues, in an interesting study, evaluated the risk factors for local progression after RFA in a series of 128 patients with lung tumors (21). This series included primarily patients with metastatic lung neoplasm. The median follow-up was 12 months; local progression was seen in 94/342 (27%) of lesions. Larger tumor size and the use of an internally cooled electrode were independent risk factors for local progression. Thus, in the future, further advances in technology or adjuvant therapy may be useful in decreasing progression after RFA, and, perhaps, in improving survival.
Limitations
The current study also has the limitations which are common to retrospective studies, such as selection bias. The patients who were treated in this study comprise a very heterogeneous group, which encompasses not only primary lung neoplasm, but also recurrent lung cancer and metastatic disease. Further, many patients had failed other therapies, and these treated tumors may represent a more aggressive tumor biology. In addition, the use of other therapies in the patients treated with RFA confounds the analysis of efficacy of treatment with RFA. We also need longer follow-up to fully evaluate survival endpoints. In addition, further prospective studies are required to definitively compare RFA with conventional external beam radiation treatment or other emerging technologies such as stereotactic radiosurgery.
Conclusion
In summary, this study is a report on the use of CT-guided radiofrequency ablation for the treatment of lung neoplasm in 100 consecutive patients by thoracic surgeons. There are several factors which should be investigated further including optimal patient selection, and measures to improve upon local control of the tumor. Surgery remains the best treatment for resectable lung cancer, however emerging technologies, such as RFA, may have a role in patients who are medically inoperable. Further prospective studies are necessary to define the role of RFA in the treatment of lung neoplasm. Image-guided RFA done by the thoracic surgeons is feasible and safe in high-risk patients with lung neoplasm with reasonable results in patients who are not fit for surgery. Thoracic surgeons can perform RFA safely, and should continue to investigate this new image-guided modality which may offer an alternative option in medically inoperable patients. Thoracic surgeons should continue to evaluate new technology and add these to their armamentarium in the treatment of lung neoplasm.
Acknowledgments
This research was funded in part by the National Institutes of Health (NIH) Specialized Program of Research Excellence in Lung Cancer (P50 CA090440), and in part by research grants from RITA Medical/Angiodynamics to the University of Pittsburgh.
Footnotes
Presented at the Society of Thoracic Surgeons, Fort Lauderdale, January 2008
References
- 1.Ginsberg RJ, Martini N. Non – Small Cell Lung Cancer/Surgical Management. In: Pearson FG, Cooper JD, Deslauriers J, Ginsberg RJ, Hiebert CA, Patterson GA, Urschel HC Jr, editors. Thoracic Surgery. 2nd. Philadelphia, PA: Churchill Livingstone; 2002. pp. 837–59. [Google Scholar]
- 2.Ginsberg RJ, Rubinstein LV, Lung Cancer Study Group Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg. 1995;60:615–623. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 3.Raz DJ, Zell JA, Ou SH, Gandara DR, Anton-Culver H, Jablons DM. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest. 2007;132(1):193–9. doi: 10.1378/chest.06-3096. [DOI] [PubMed] [Google Scholar]
- 4.McGarry RC, Song G, des Rosiers P, et al. Observation-only management of early stage, medically inoperable lung cancer: poor outcome. Chest. 2002;121:1155–1158. doi: 10.1378/chest.121.4.1155. [DOI] [PubMed] [Google Scholar]
- 5.Jeremic B, Classen J, Bamberg M. Radiotherapy alone in technically inoperable, medically inoperable, early stage (I/II) non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;54(1):119–30. doi: 10.1016/s0360-3016(02)02917-6. [DOI] [PubMed] [Google Scholar]
- 6.Sibley G, Jamieson T, Marks L, et al. Radiotherapy alone for medically inoperable stage I non–small-cell lung cancer: The Duke experience. Int J Radiat Oncol Biol Phys. 1998;40:149–54. doi: 10.1016/s0360-3016(97)00589-0. [DOI] [PubMed] [Google Scholar]
- 7.Kaskowitz L, Graham MV, Emami B, et al. Radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1993;27:517–23. doi: 10.1016/0360-3016(93)90374-5. [DOI] [PubMed] [Google Scholar]
- 8.Qaio X, Tullgren O, Lax I, Sirzen F, Lewensohn The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer. 2003;41:1–11. doi: 10.1016/s0169-5002(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 9.Kupelian PA, Komaki R, Allen P. Prognostic factors in the treatment of node-negative non–small cell lung carcinoma with radiotherapy alone. Int J Radiat Oncol Biol Phys. 1996;36:607–613. doi: 10.1016/s0360-3016(96)00364-1. [DOI] [PubMed] [Google Scholar]
- 10.Pastorino U, Buyse M, Friedel G, et al. The International Registry of Lung Metastases. Long-term results of lung metastasectomy prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 11.Thomsen S. Radio Frequency Ablation of Thoracic malignancies. In: Franco K, Putnam J, editors. Advanced Therapy in Thoracic Surgery. 2nd. BC Decker Inc; Hamilton, Ontario: 2005. pp. 75–90. [Google Scholar]
- 12.Ferguson MK. Preoperative assessment of pulmonary risk. Chest. 1999;115:58S–63S. doi: 10.1378/chest.115.suppl_2.58s. [DOI] [PubMed] [Google Scholar]
- 13.Eagle KA, Berger PB, Calkins H, et al. Circulation. 10. Vol. 105. 2002. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery---executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) pp. 1257–1267. [PubMed] [Google Scholar]
- 14.Herrera LJ, Fernando HC, Perry Y, et al. Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg. 2003;125:929–37. doi: 10.1067/mtc.2003.18. [DOI] [PubMed] [Google Scholar]
- 15.Fernando HC, De Hoyos A, Landreneau RJ, et al. Radiofrequency ablation for the treatment of non-small cell lung cancer in marginal surgical candidates. J Thorac Cardiovasc Surg. 2005;129:639–44. doi: 10.1016/j.jtcvs.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Sugimura H, Nichols FC, Yang P, et al. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg. 2007;83:409–18. doi: 10.1016/j.athoracsur.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 18.Whyte RI, Crownover R, Murphy MJ, et al. Stereotactic radiosurgery for lung tumors preliminary report of a phase I trial. Ann Thorac Surg. 2003;75:1097–1101. doi: 10.1016/s0003-4975(02)04681-7. [DOI] [PubMed] [Google Scholar]
- 19.Yan TD, King J, Sjarif A, Glenn D, Steinke K, Morris DL. Percutaneous radiofrequency ablation of pulmonary metastases from colorectal carcinoma: prognostic determinants for survival. Ann Surg Oncol. 2006;13(11):1529–37. doi: 10.1245/s10434-006-9101-1. [DOI] [PubMed] [Google Scholar]
- 20.Yan TD, King J, Sjarif A, et al. Treatment Failure after Percutaneous Radiofrequency Ablation for Nonsurgical Candidates with Pulmonary Metastases from Colorectal Carcinoma. Ann Surg Oncol. 2007;14:1718–26. doi: 10.1245/s10434-006-9271-x. [DOI] [PubMed] [Google Scholar]
- 21.Hiraki T, Sakurai J, Tsuda T, et al. Risk factors for local progression after percutaneous radiofrequency ablation of lung tumors. Evaluation based on a preliminary review of 342 tumors. Cancer. 2006;12:107. 2873–2880. doi: 10.1002/cncr.22333. [DOI] [PubMed] [Google Scholar]
- 22.Ambrogi MC, Lucchi M, Dini P, et al. Percutaneous radiofrequency ablation of lung tumours: results in the mid-term. Eur J Cardiothorac Surg. 2006;30:177–83. doi: 10.1016/j.ejcts.2006.03.067. [DOI] [PubMed] [Google Scholar]
- 23.Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–275. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 24.Birim O, Maat APWM, Kappetein AP, et al. Validation of the Charlson comorbidity index in patients with operated primary nonsmall cell lung cancer. Eur J Cardiothorac Surg. 2003;23:30–34. doi: 10.1016/s1010-7940(02)00721-2. [DOI] [PubMed] [Google Scholar]
- 25.Choong C, Meyers BF, Battafarano RJ, et al. Lung cancer resection combined with lung volume reduction in patients with severe emphysema. J Thorac Cardiovasc Surg. 2004;127:1323–31. doi: 10.1016/j.jtcvs.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 26.Hiraki T, Gobara H, Iishi T, et al. Percutaneous radiofrequency ablation for clinical stage I non–small cell lung cancer: Results in 20 nonsurgical candidates. J Thorac Cardiovasc Surg. 2007;134:1306–12. doi: 10.1016/j.jtcvs.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 27.El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol. 2007;14:2400–5. doi: 10.1245/s10434-007-9421-9. [DOI] [PubMed] [Google Scholar]
- 28.Lee JM, Youk JH, Kim YK, et al. Radio-frequency thermal ablation with hypertonic saline solution injection of the lung: ex vivo and in vivo feasibility studies. Eur Radiol. 2003;13:2540–2547. doi: 10.1007/s00330-003-1876-x. [DOI] [PubMed] [Google Scholar]