Table 2.
α-Selective Ni-Catalyzed Hydroalumination of Arylacetylenesa
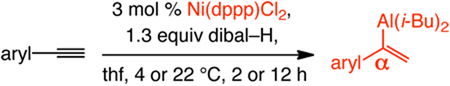 | |||||
|---|---|---|---|---|---|
| entry | aryl | temp (°C) | time (h)b | conv (%)c | α:βc |
| 1 | o-OMeC6H4 | 4 | 12 | >98 | 98:2 |
| 2 | m-OMeC6H4 | 22 | 2 | >98 | >98:2 |
| 3 | p-OMeC6H4 | 4 | 12 | >98 | >98:2 |
| 4 | m-CF3C6H4 | 4 | 12 | >98 | 95:5 |
| 5 | p-CF3C6H4 | 22 | 2 | >98 | 97:3 |
| 6 | p-FC6H4 | 22 | 2 | >98 | >98:2 |
| 7 | o-ClC6H4 | 22 | 2 | >98 | >98:2 |
| 8 | o-BrC6H4 | 22 | 2 | >98 | >98:2 |
| 9 | o-MeC6H4 | 4 | 12 | >98 | >98:2 |
| 10 | 3-pyridyl | 22 | 2 | >98 | >98:2 |
| 11 | 3-pyridyl | 22 | 2 | >98 | >98:2 |
Reactions under N2 atm.
Reaction times correspond to hydroalumination portion of the process (not including D2O quench).
By analysis of 400 MHz 1H NMR spectra of unpurified mixtures (after D2O); <2% alkynylaluminum observed.
