Figure 2.
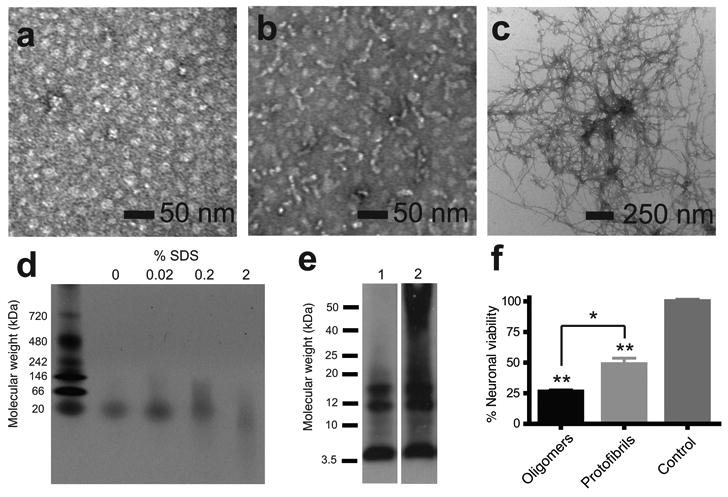
Characterization of Aβ42 oligomers, protofibrils and fibrils. (a) TEM of Aβ42 oligomers incubated at 4 °C for 6 h. (b) TEM of Aβ42 protofibrils incubated at 37 °C for 6 h; (c) TEM of Aβ42 fibrils incubated at 37 °C for 12 days. (d) Native gel electrophoresis showing that oligomers contain a single band at ∼20 kDa and that increasing the SDS content can disrupt the oligomeric conformation. (e) Sodium dodecylsulfate (SDS)-polyacrylamide gel electrophoresis of Aβ42 oligomers incubated at 4 °C for 6 h (lane 1) and protofibrils incubated at 37 °C for 6 h (lane 2). Aβ42 was detected using the monoclonal 6E10 anti-Aβ antibody. (f) Cell viability assay of primary cultures of murine cortical neurons treated with Aβ42 oligomers incubated at 4 °C for 6 h or protofibrils incubated at 37 °C for 6 h (n=5). Results represent the mean ± sem, * p < 0.02, ** p <0.001.
