Figure 4.
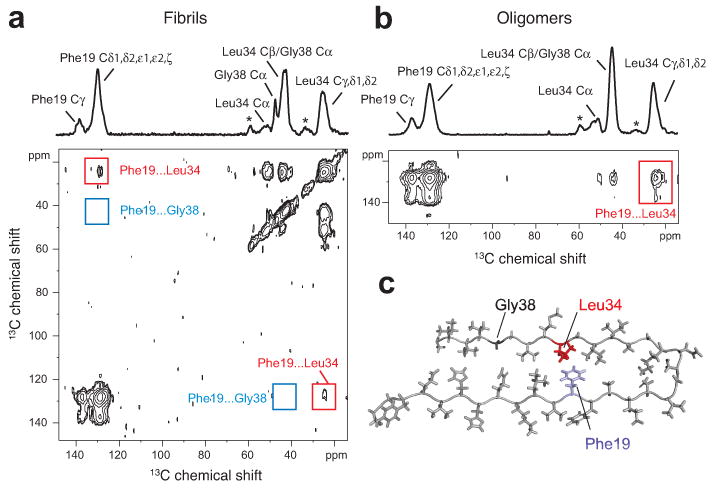
Turn structure in Aβ42 fibrils and neurotoxic oligomers. (a) Above, one-dimensional 13C-NMR spectrum showing chemical shift assignments for Phe19, Leu34 and Gly38 in Aβ42 fibrils formed from Aβ42-FLG peptides. Natural abundance (na) cross-peaks are marked with an asterisk. Below, region of the two-dimensional DARR NMR spectrum showing specific 13C…13C molecular contacts between Phe19 and Leu34 (red box) and no contact between Phe19 and Gly38 (blue box) in Aβ42 fibrils. (b) Above, one dimensional 13C-spectrum showing chemical shift assignments for Phe19, Leu34, and Gly38 in Aβ42 oligomers formed from Aβ42-FLG peptides. Below, two-dimensional DARR NMR spectrum showing a specific 13C…13C contact between Phe19 and Leu34 in Aβ42 oligomers. Due to the chemical shift overlap between the Cα of Gly38 and the Cβ of Leu34 in the oligomers, we cannot rule out Phe19-Gly38 contacts in a minor population of Aβ42 peptides. (c) Molecular model of the turn conformation in Aβ42 highlighting the Phe19-Leu34 contact (see also Supplementary Results 1).
