Figure 3.
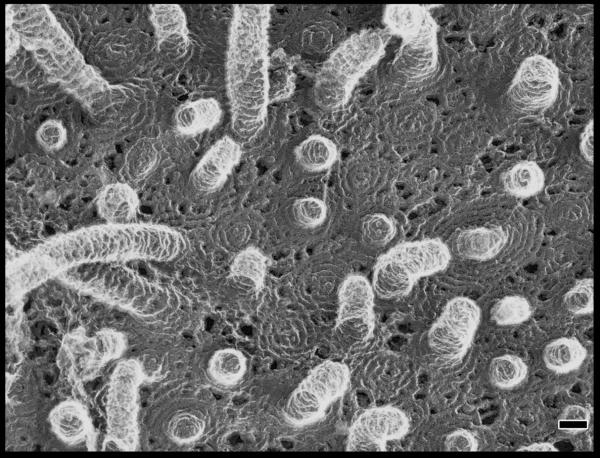
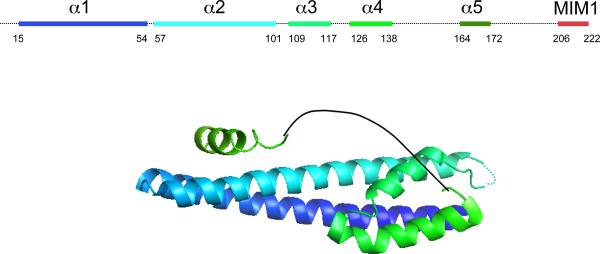
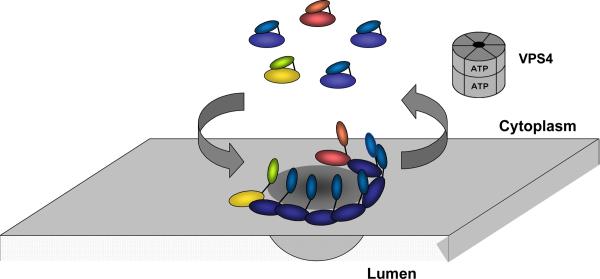
ESCRT-III. a. Assembled ESCRT-III polymer is visualized in this electron micrograph showing the filaments underlying buds and tubules emerging from the top of a cell overexpressing human SNF7 and an inactive mutant of VPS4B. Image is reproduced, with permission, from21. The SNF7-containing filaments are seen because of post-fixation extraction with detergent. b. Helical organization of individual ESCRT-III proteins based on the observed structure of human VPS2433,58. Helices α1– α4 form the core of the protein responsible for membrane binding and polymer assembly and are regulated by autoinhibitory sequences in α5 and the C-terminal MIM1 (not resolved in the CHMP3 structure). All ESCRT-III proteins contain the α1– α4 core and the α5 autoinhibitory sequence. Vps2, Did2, and Vps24 contain a C-terminal MIM1 motif, whereas Vps20 and Snf7 contain a C-terminal MIM2 motif. Ist1 has both MIM1 and MIM2 motifs at its C-terminus. Both types of MIM bind to the MIT domain of Vps4, but they do so at separate sites and do not compete with one another. c. Cycle of ESCRT-III assembly and VPS4-mediated disassembly. ESCRT-III proteins are in a `closed' and monomeric conformation in the cytosol, with intramolecular autoinhibitory interactions preventing membrane binding and polymer assembly. In their `open' conformation, these autoinhibitory interactions are released, and the subunits bind to each other and to the membrane as they assemble into functional ESCRT-III polymers.
-This could be redrawn with colors that fit better w/ rest of figs