Abstract
Susceptibility to multiple sclerosis is higher in females than males. However the underlying mechanism behind this gender difference is poorly understood. Because the presence of neuroantigen-primed T cells in the CNS is necessary to initiate the neuroinflammatory cascade of MS, we first investigated how these T cells interacted with astroglia, major resident glial cells of the CNS. Interestingly, we found that myelin basic protein-primed T cells from female and castrated male mice, but not from male mice, produced proinflammatory molecules like NO, IL-1β and IL-6 in astroglia and these responses were purely via contact between T cells and astroglia. Because T cell:glia contact requires several integrin molecules, we examined the involvement of integrins in this process. Both α4 and β1, subunits of VLA-4 integrin, were found to be necessary for T cell contact-induced generation of proinflammatory molecules in astroglia. Interestingly, the expression of β1, but not α4, was absent in male MBP-primed T cells. On the other hand, female and castrated male MBP-primed T cells expressed both α4 and β1. Similarly we also detected β1 in spleen of normal young female, but not male, mice. Furthermore, we show that male sex hormones (testosterone and dihydrotestosterone), but not female sex hormones (estrogen and progesterone), were able to suppress the mRNA expression of β1 in female MBP-primed T cells. These studies suggest that β1, but not α4, integrin of VLA4 is the sex-specific molecule on T cell surface and that the presence or absence of β1 determines gender-specific T cell contact-mediated glial activation.
Keywords: Proinflammatory molecules, Gender, VLA4, β1 integrin, Sex hormones
Introduction
Multiple sclerosis (MS) is the most common human autoimmune demyelinating disease of the central nervous system (CNS). It has been known for decades that a female is twice as likely as a male to be affected from MS. This is evident from the fact that about 66 % of MS patients are female (1, 2). Female prevalence is not only observed in MS but also in other autoimmune diseases like Addison’s, rheumatoid arthritis, pernicious anemia, Sjogren’s, systemic lupus erythematosus, and thyroiditis (3). The corresponding animal models of these diseases including experimental allergic encephalomyelitis (EAE), an animal model of MS, also exhibit female biasness (4–7). Although hormonal and genetic factors have been postulated (8), the molecular mechanism behind this gender bias is still poorly understood.
The hallmark of brain inflammation in MS is the activation of glial cells that express and produce a variety of proinflammatory and neurotoxic molecules, including inducible nitric oxide synthase (iNOS) and proinflammatory cytokines (9–13). Semiquantitative RT-PCR for iNOS mRNA in MS brains shows markedly higher expression of iNOS mRNA in MS brains than in normal brains (14, 15). Hooper et al (16) have reported that uric acid, a scavenger of peroxynitrite (a highly reactive derivative of NO), markedly inhibits the appearance of EAE in mice and that the incidence of MS is very rare among gout patients having higher levels of uric acid. Among proinflammatory cytokines, primary inflammatory cytokines, such as interleukin (IL)-1β/α, tumor necrosis factor (TNF)α/β, and IL-6, play a predominant role since they are involved at multiple levels of neuroimmune regulation (13, 17, 18). Analysis of cerebrospinal fluid (CSF) from MS patients has shown increased levels of proinflammatory cytokines compared with normal control, and levels of those cytokines in the CSF of MS patients also correlate with disease severity (19). Consistently, blockade of proinflammatory cytokine synthesis or function by signaling inhibitors or neutralizing antibodies or gene knockout can also prevent the development of EAE (18, 20). However, the mechanisms by which these proinflammatory molecules are produced in the CNS of MS patients are poorly understood.
Recently we have observed that neuroantigen-specific T cells induce microglial expression of iNOS and proinflammatory cytokines (IL-1β, IL-1α, TNF-α and IL-6) through very-late antigen-4 (VLA4)-mediated cell-to-cell contact (21). Activation of both NF-κB and C/EBPβ was involved in T cell contact-mediated microglial activation (21). However, VLA4-mediated contact was responsible for microglial activation of C/EBPβ, but not NF-κB (21). When we examined the gender-dependency of this response, we found that MBP-primed T cells isolated from female and castrated male mice, but not male mice, induced the expression of proinflammatory molecules (iNOS, IL-1β, IL-1α, IL-6 and TNF-α) in microglia via cell-to-cell contact (22). Interestingly, T cell contact-mediated microglial activation of C/EBPβ, but not NF-κB, was gender sensitive (22). Taken together, these results suggest that VLA4 integrin on T cell surface could be the gender specific molecule regulating gender-specific activation of microglial C/EBPβ by T cell contact.
Due to the facts that astroglia constitute the majority of resident glial cells outclassing neuron and microglia by huge margin of population and that astroglial activation also contributes significantly to overall CNS inflammation (23–26), we tried to unravel the mystery further behind gender biasness of neuroantigen-specific T cell contact-mediated glial activation using primary mouse astroglia. Here we report that female and castrated male, but not male, MBP-specific T cells induce the expression of proinflammatory molecules in astroglia via cell-to-cell contact. VLA4 is a heterodimer of α4 and (β1 integrins. Interestingly, MBP-primed T cells of female, male and castrated male mice expressed α4 integrin of VLA4. In contrast, MBP-primed T cells of female and castrated male mice, but not that of male mice, expressed β1 integrin. Furthermore, we demonstrate that male (testosterone and DHT), but not female, sex hormones (estrogen and progesterone) are capable of suppressing the expression of β1 in MBP-specific T cells. These studies identify β1 integrin of VLA4 as a gender-specific molecule on T cell surface dictating the gender-specific T cell function.
MATERIALS AND METHODS
Reagents
Fetal bovine serum, Hank's balanced salt solution (HBSS), DMEM/F-12, RPMI 1640, L-glutamine, and β-mercaptoethanol were from Mediatech. Assay systems for IL-1β and IL-6 were purchased from BD Pharmingen. Bovine myelin basic protein was purchased from Invitrogen. Functional blocking antibodies and FITC-labeled antibodies to CD49d (the α4 chain of VLA-4) and CD29 (the β1 chain of VLA-4) were obtained from Pharmingen. PE-labeled antibody to CD3 was purchased from eBioscience. Multigene-12 RT-PCR profiling kits for mouse integrin gene family I & II were purchased from SuperArray Bioscience Corporation. Annexin V-PE apoptosis detection kit was obtained from Biovision. β-estradiol, progesterone, testosterone and dihydrotestosterone (5α-androstan-17β-ol-3-one) were purchased from Sigma.
Isolation of MBP-primed T Cells
Specific pathogen-free female, male, and castrated male SJL/J mice (4 – 6 weeks old) were purchased from Harlan Sprague-Dawley, Inc. MBP-primed T cells were isolated and purified as described earlier (21, 22). Briefly, mice were immunized subcutaneously with 400 µg of bovine MBP and 60 µg Mycobacterium tuberculosis (H37RA, Difco Laboratories) in incomplete Freund's adjuvant (IFA) (Calbiochem). Lymph nodes and spleens were collected from these mice, and single cell suspension was prepared in RPMI 1640 medium containing 10% fetal bovine serum, 2 mM L-glutamine, 50 µM β-mercaptoethanol, 100 units/ml penicillin, and 100 µg/ml streptomycin. Cells were cultured at a concentration of 4–5 × 106 cells/ml in twelve-well plates. Cells isolated from MBP-immunized mice were incubated with 50 µg/ml MBP for 4 days. The non-adherent cells were used to stimulate astroglial cells.
Passive transfer of MBP-primed T cells
Donor mice were immunized s.c. with 400 µg bovine MBP and 60 µg Mycobacterium tuberculosis in IFA (16). Animals were killed 10–12 days postimmunization, and the draining lymph nodes were harvested. Single-cell suspensions were treated with RBC lysis buffer (Sigma-Aldrich), washed, and cultured at a concentration of 4–5 × 106 cells/ml in 6-well plates in RPMI 1640 supplemented with 10% FBS, 50 µg/ml MBP, 50 µM 2-ME, 2 mM l-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. On day 4, cells were harvested and resuspended in HBSS. A total of 2 × 107 viable cells in a volume of 200 µl was injected into the tail vein of naive mice. Pertussis toxin (150 ng/mouse; Sigma-Aldrich) was injected once via i.p. route on 0 days posttransfer (dpt) of cells. Cells isolated from donor mice immunized with CFA or IFA alone were not viable after 4 days in culture with MBP and therefore were not transferred.
Isolation of Mouse Primary Astroglia
Astroglia were isolated from mixed glial cultures following the procedure of Giulian and Baker (1986) (27) as described previously (28). Briefly, cerebra taken from 2- to 3-d-old mouse pups were chopped, triturated, passed through mesh, and trypsinized for the isolation of mixed glial cells. On day 9, the mixed glial cultures were washed three times with DMEM/F-12 and subjected to a shake at 240 rpm for 2 h at 37°C on a rotary shaker to remove microglia. Similarly, on day 11, cells were shaken at 180 rpm for 18 h to remove oligodendroglia. Then, attached cells, primarily the astroglia, were trypsinized, subcultured and plated accordingly to our experimental requirements.
Preparation of Plasma Membrane
Plasma membranes of MBP-primed T cells were prepared by sonication and centrifugation. Briefly, the cells were broken up by sonication, and the nuclear fraction was discarded after centrifugation for 10 min at 4000g. The supernatant was centrifuged for 45 min at 100,000g. The pellet of T cell membranes was resuspended at 50 × 106 cell equivalents/ml by sonication in HBSS containing 20 µM EDTA and 5 µM iodoacetamide.
Stimulation of Mouse Primary Astroglia by MBP-primed T Cells
Astroglial cells were stimulated with different concentrations of MBP-primed T cells under serum-free condition. After 1h of incubation, culture dishes were shaken and washed thrice with HBSS to lower the concentration of T cells. Earlier, by fluorescence-activated cell sorting analysis of adherent microglial cells using fluorescein isothiocyanate-labeled anti-CD3 antibodies, we demonstrated that more than 80% T cells were removed from microglial cells by this procedure (21). Then astroglial cells were incubated in serum-free media for different periods of time depending on the experimental requirements.
Assay for NO Synthesis
Synthesis of NO was determined by assay of culture supernatants for nitrite, a stable reaction product of NO with molecular oxygen. Briefly, supernatants were centrifuged to remove cells, and 400 µl of each supernatant was allowed to react with 400 µl of Griess reagent (29, 30) and incubated at room temperature for 15 min. The optical density of the assay samples was measured spectrophotometrically at 570 nm. Fresh culture media served as the blank. Nitrite concentrations were calculated from a standard curve derived from the reaction of NaNO2 in the assay.
Assay for IL-1β and IL-6 Synthesis
Concentrations of IL-1β and IL-6 were measured in culture supernatants by a high-sensitivity enzyme-linked immunosorbent assay (BD Pharmingen) according to the manufacturer's instruction as described earlier (31).
Semi-quantitative RT-PCR Analysis
Total RNA was isolated from cells by using RNeasy mini kit (Qiagen) and from spleen by using Ultraspec-II RNA reagent (Biotecx laboratories, Inc) following manufacturer’s protocol. To remove any contaminating genomic DNA, total RNA was digested with DNase. Semi-quantitative RT-PCR was carried out as described earlier (32, 33) using a RT-PCR kit from clonetech. Briefly, 1 µg of total RNA was reverse transcribed using oligo (dT)12–18 as primer and MMLV reverse transcriptase (Clontech) in a 20 µl reaction mixture. The resulting cDNA was appropriately-diluted, and diluted cDNA was amplified using Titanium Taq DNA polymerase and following primers. Amplified products were electrophoresed on a 1.8% agarose gels and visualized by ethidium bromide staining.
| iNOS: | Sense: 5’-CCCTTCCGAAGTTTCTGGCAGCAGC-3’ |
| Antisense: 5’-GGCTGTCAGAGCCTCGTGGCTTTGG3’ | |
| IL-1β: | Sense: 5’-CTCCATGAGCTTTGTACAAGG-3’ |
| Antisense: 5’-TGCTGATGTACCAGTTGGGG-3’ | |
| IL-6: | Sense: 5’-GACAACTTTGGCATTGTGG -3’ |
| Antisense: 5’-ATGCAGGGATGATGTTCTG-3’ | |
| Integrin β1: | Sense: 5’-GAGACATGTCAGACCTGCCTTGGCG-3’ |
| Antisense: 5’-GGGATGATGTGGGGACCAGTAGGAC-3’ | |
| Integrin α4: | Sense: 5’-AACCGGGCACTCCTACAACCTGGAC- 3’ |
| Antisense: 5’-ACCCCCAGCCACTGGTTATCCCTCT- 3’ | |
| Integrin β2: | Sense: 5’-CTGCTGTGTCCCAGGAATGCACC- 3’ |
| Antisense: 5’- CCCGCCCAGCTTCTTGACGTTGT- 3’ | |
| Integrin β7: | Sense: 5’-CTGAACTTCACTGCCTCGGGAGAGG- 3’ |
| Antisense: 5’- CTAGCTGGCGCACACGTTCCAAGTC- 3’ | |
| GAPDH: | Sense: 5’-GGTGAAGGTCGGTGTGAACG3’ |
| Antisense: 5’-TTGGCTCCACCCTTCAAGTG-3’ |
Real-time PCR Analysis
It was performed using the ABI-Prism7700 sequence detection system (Applied Biosystems) as described earlier (32). All primers and FAM-labeled probes for mouse genes and GAPDH were obtained from Applied Biosystems. The mRNA expressions of respective genes were normalized to the level of GAPDH mRNA. Data were processed by the ABI Sequence Detection System 1.6 software and analyzed by ANOVA.
Flow cytometry
Surface expression of α4 and β1 and the surface expression of β1 along with CD3 on MBP-primed T cells or apoptosis of MBP-primed T cells were monitored by single-color and two-color flow cytometry respectively as described previously (34, 35). Approximately 1 × 106 cells suspended in RPMI 1640 medium-FBS were incubated in the dark with appropriately diluted FITC-labeled antibodies to CD49d (integrin α4 chain) or CD29 (integrin β1 chain) for single color at 4 °C for 1 hr. For two-color, 1 × 106 cells suspended in 1X binding buffer were incubated under the same condition with appropriately diluted FITC-labeled antibodies to β1 and PE-labeled CD3 or Annexin V-PE. Following incubation, cell suspension was centrifuged, washed three times, and resuspended in 500 µl of RPMI 1640 medium-FBS for single color or 1X PBS for two-color. The cells were then analyzed through FACS (BD Biosciences) present in the University of Rush Flow facility. A minimum of 10,000 cells was accepted for FACS analysis. Cells were gated based on morphological characteristics. Apoptotic and necrotic cells were not accepted for FACS analysis.
Analysis of Mouse Integrin α and β Gene Families by Gene-Array
Expression of different integrins was analyzed in MBP-primed T cells by a RT-PCR-based gene array kit (GEArray™) from SuperArray, Inc. following manufacturer’s protocol. Briefly, the lyophilized component of HotStart “Sweet” PCR master mix was resuspended in 300 µl of double-distilled water. Then 20 µl of each cDNA synthesis reaction product was transferred to separate tube of master mix. Twenty-five µl of a single PCR cocktail was then dispensed to each of the 12 PCR tubes of the same Multigene-12™ Primer Strip. Strips were next placed in the thermal cycler block and the appropriate program was run. Amplified products were electrophoresed on a 4% agarose gels and visualized by ethidium bromide staining.
Immunofluoroscence Analysis
Immunofluorescence analysis was performed as described earlier (24). Briefly, mice were perfused intracardially with PBS (pH 7.4), and then with 4 % (w/v) paraformaldehyde solution in PBS. Dissected spleens and cerebellum were post-fixed in 4 % formaldehyde/PBS for 2–5 days and cryoprotected in 20 % sucrose/PBS overnight at 4 C. Tissues were then embedded in OCT (TissueTek, Elkhart, IN) at −50 C, and processed for conventional cryosectioning to obtain frozen longitudinal sections (8 µm) and stored at −80 C. Frozen sections were then allowed to cool to at room temperature for 1.5 – 2 h, washed six times each for 5 minutes in 1X PBS, blocked in 2 % BSA in 1 X PBS with 0.5 % Triton at room temperature and incubated with rat anti-integrin β1 (1:400) (Chemicon) and goat anti-CD3 (1:100) (eBioscience) Abs for overnight at room temperature for dual immunohistochemistry. Sections were then washed six times in 1X PBS and further incubated with Cy2 and Cy5 (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1.5 h at room temperature followed by overnight drying. Next, the sections were rinsed in distilled water, dehydrated successively in ethanol and xylene and mounted and observed under an Olympus fluorescence microscope using a 40X objective.
RESULTS
MBP-primed T cells Isolated from Female and Castrated Male, but not Male, SJL/J Mice Induced the Expression of iNOS, IL-1β and IL-6 in Mouse Primary Astroglia via Cell-to-Cell Contact
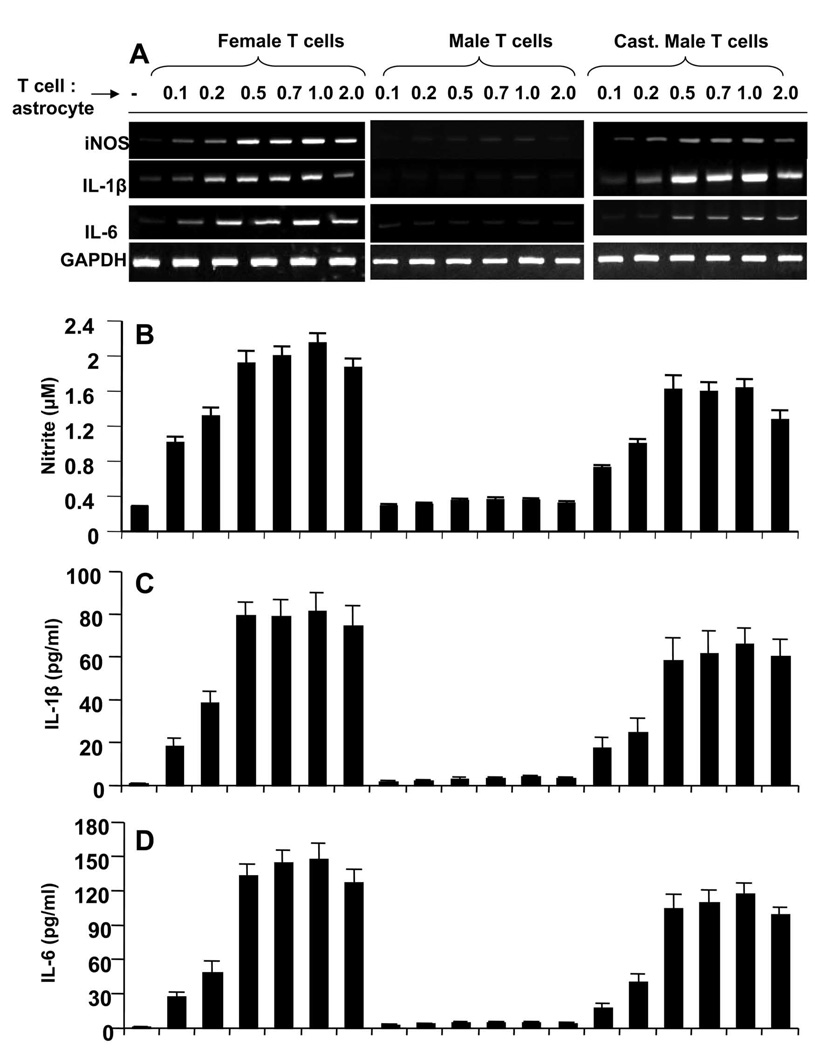
Earlier we have noticed that MBP-primed T cell contact-induced expression of proinflammatory molecules in microglia is gender sensitive (21, 22). Because astroglia are major glial cells in the CNS, we investigated whether astroglial production of proinflammatory molecules via T cell contact has also gender specificity. As described in earlier studies (21, 22), MBP-primed T cells were washed and added to mouse primary astroglia in direct contact. After 1 h of contact, culture dishes were shaken and washed thrice to remove MBP-primed T cells. We found that astroglia also responded in a similar fashion like microglia to MBP-primed T cells (21, 22). MBP-primed T cells of female mice markedly induced the expression of proinflammatory molecules (iNOS, IL-1β and IL-6) in astroglia at different ratios of T cell:glia with the maximum increase found at 0.5:1 or 1:1 of T cell: glia (Fig. 1A). Nitrite estimation and ELISA assay of supernatants also show that female MBP-primed T cells induced the production of NO (Fig. 1B), IL-1β (Fig. 1C) and IL-6 (Fig. 1D) in mouse primary astroglia. However, unlike female MBP-primed T cells, MBP-primed T cells isolated from male mice were unable to induce the expression of proinflammatory molecules (Fig. 1A) and the production of NO, IL-1β and IL-6 (Fig. 1B–D) in astroglia suggesting the possible involvement of male sex hormone in disabling male MBP-primed T cells from contact-mediated activation of astroglia. To further establish this hypothesis, we isolated MBP-primed T cells from castrated male mice. Interestingly, after castration, MBP-primed T cells from male mice behaved similar to female MBP-primed T cells and induced the mRNA expression of iNOS, IL-1β and IL-6 (Fig. 1A) and the production of NO, IL-1β and IL-6 proteins (Fig. 1B–D) in astroglia.
Figure 1. MBP-primed T cells from female and castrated male, but not male, SJL/J mice induce the expression of proinflammatory molecules in primary mouse astroglia.
Primary astroglia received different concentrations of MBP-primed T cells of female, male and castrated male mice in direct contact under serum-free condition. After 1 h of incubation, culture dishes were shaken and washed thrice with HBSS to remove burden of T cells. A, then adherent astroglial cells were incubated in serum-free media for 5 h and expressions of iNOS, IL-1β and IL-6 were analyzed by semi-quantitative RT-PCR. After removal of T cells, adherent astroglial cells were incubated in serum-free media for 23 h and supernatants were used to assay nitrite (B), IL-1β (C) and IL-6 (D) as described in “Materials and Methods”. Data are mean ± S.D. of three different experiments.
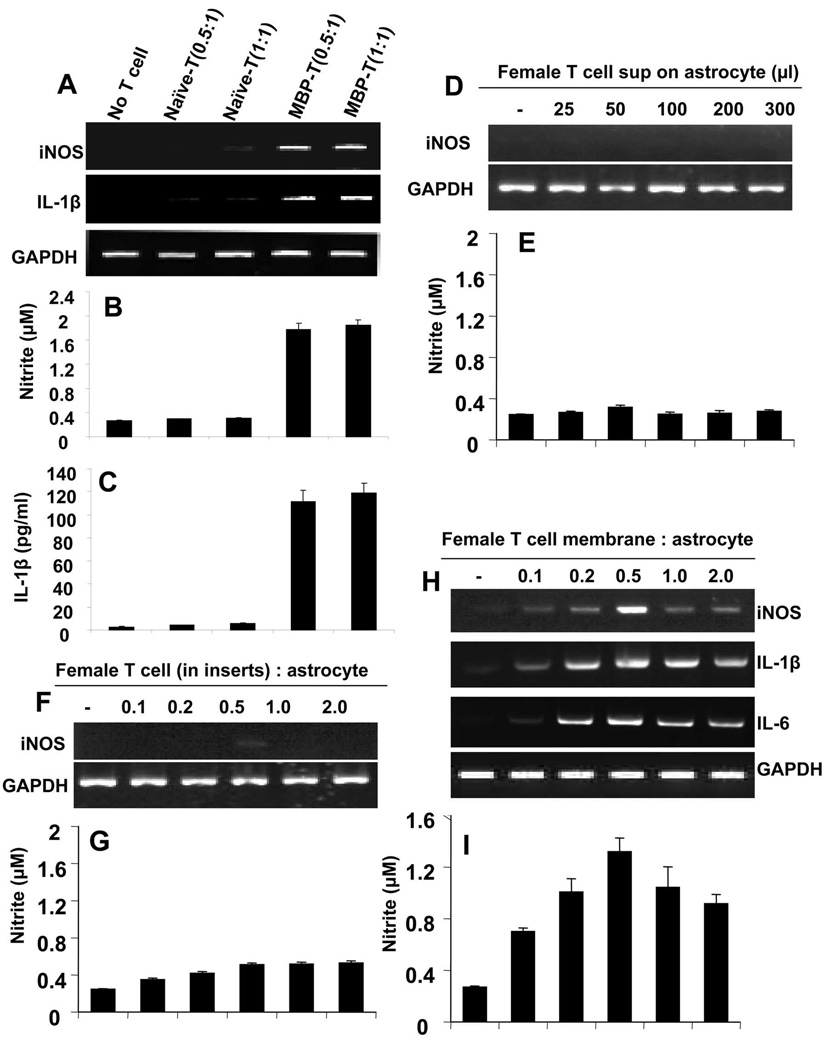
To examine whether priming of T cells with antigen is necessary to induce proinflammatory molecules in astroglia, different doses of naïve and MBP-primed T cells from female mice were added to mouse primary astroglia in direct contact. Our results (Fig. 2A to C) clearly demonstrate that, only MBP-primed, but not naïve, T cells were able to induce the mRNA expressions of iNOS and IL-1β (Fig. 2A), and produce NO (Fig. 2B) and IL-1β (Fig. 2C), suggesting that antigen priming of T cells is necessary to elicit proinflammatory responses in astroglia.
Figure 2. MBP-primed, but not naïve, T cells induce the expression of proinflammatory molecules in primary mouse astroglia via cell-to-cell contact.
Primary astroglia received different concentrations of naïve or MBP-primed T cells of female mice in direct contact under serum-free condition. After 1 h of incubation, culture dishes were shaken and washed thrice with HBSS to remove burden of T cells. After 5 h incubation with serum-free media, astroglia were analyzed for expression of iNOS and IL-1β by semi-quantitative RT-PCR (A) and after 24 h of incubation, supernatents were used for nitrite (B) and ELISA assay (C). Primary astroglia received different concentrations of conditioned supernatants of female MBP-primed T cells under serum-free condition. After 6 h of incubation, adherent astroglia were analyzed for expression of iNOS by semi-quantitative RT-PCR (D) and after 24 h of incubation, supernatants were used to assay nitrite (E). Astroglia received different concentrations of female MBP-primed T cells within insert under serum-free condition. After 6 h of incubation, adherent astroglia were analyzed for expression of iNOS by semi-quantitative RT-PCR (F) and after 24 h of incubation, supernatants were used to assay nitrite (G). After cell counting, female MBP-primed T cells were subjected to plasma membrane preparation as mentioned under “Materials and Methods”. Then astroglia were incubated with plasma membranes of MBP-primed T cells under serum-free condition. After 6 h of incubation, astroglia were analyzed for expression of iNOS, IL-1β and IL-6 by semi-quantitative RT-PCR (H) and after 24 h of incubation, supernatants were used to assay nitrite (I).
Next we examined whether contact was necessary for the induction of proinflammatory molecules in astroglia. Therefore, at first, we used conditioned supernatants of T cells to investigate the role of soluble factors released from T cells. It is clear from figure 2D and 2E that different amount of supernatants was unable to induce the expression of iNOS and the production of NO in astroglia. Here we must mention that 50 µl of supernatant was equivalent to T cells for 0.5:1 of T cell:astroglia. Then T cells were put in inserts, so that the cells were maintained in close proximity to astroglia but the actual contact between them was shut off. In contrast to marked induction of iNOS, IL-1β and IL-6 by T cell:astroglia contact (Fig. 1), no significant increase either in the expression of iNOS (Fig. 2F) or in the production of NO (Fig. 2G) was observed. These results suggest that direct contact between T cells and astroglia is essential for the induction of iNOS and proinflammatory cytokines in astroglia. To show that direct contact is sufficient to induce the expression of these proinflammatory molecules in astroglia, membranes of MBP-primed T cells were prepared and added on astroglia in equivalents amounts of T cell:astroglia. As expected, there was gradual increase in the expression of iNOS, IL-1β and IL-6 mRNAs (Fig. 2H) as well as the production of NO (Fig. 2I) by plasma membrane of female MBP-primed T cells. These observations strongly suggest that MBP-primed T cell contact is sufficient to induce the expression of proinflammatory molecules in astroglia.
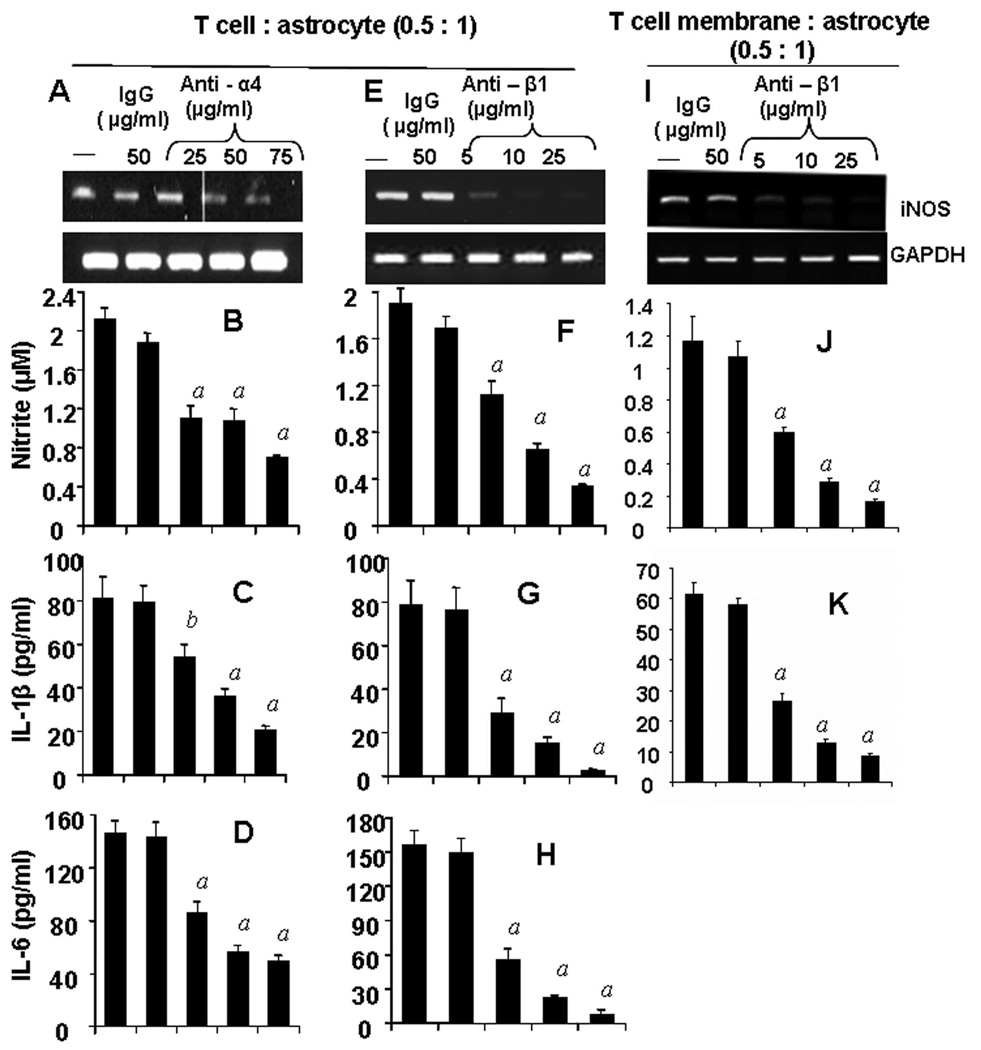
Functional Blocking Antibodies Against Subunits of VLA-4 Inhibited the Ability of Female MBP-primed T cells to Induce Contact-mediated Production of Proinflammatory Molecules in Mouse Primary Astroglia
Earlier we have shown that α4 integrin of VLA4 on the surface of MBP-primed T cells plays an important role in T cell contact-mediated activation of microglia (21, 22). Therefore, we examined if this molecule was also involved in T cell contact-mediated induction of proinflammatory factors in astroglia. We blocked α4 and β1 subunits one at a time by using functional blocking antibodies against these subunits. Although not very effective at low concentration (25 µg/ml), blocking of α4 chain by antibodies at the concentration of 50 & 75 µg/ml significantly inhibited the expression of iNOS mRNA (Fig. 3A) and the production of proinflammatory molecules (NO, IL-1β and IL-6) (Fig. 3B–D) in mouse primary astroglia. Interestingly, functional blocking of β1 was more effective than that of α4 in negating the contact activity of MBP-primed T cells. A concentration of 5 µg/ml of anti-β1 antibody was sufficient to inhibit the T cell contact-mediated expression of iNOS mRNA (Fig. 3E) and the production of proinflammatory molecules (Fig. 3F–H) in astroglia. Almost complete inhibition of proinflammatory molecule production was observed at a concentration of 25 µg/ml anti-β1 antibody (Fig. 3E–H). These observations suggest that both α4 and β1 chain of VLA-4 is necessary for contact-mediated induction of proinflammatory molecules in astroglia. Similar result was observed when β1 subunit was blocked in membrane fraction instead of whole cells (Fig. 3I–K), confirming that VLA-4 is essential for contact-mediated induction of proinflammatory molecules in astroglia.
Figure 3. Functional blocking antibodies against either α4 or β1 chain of VLA-4 inhibit the ability of female MBP-primed T cells to induce the expression of proinflammatory molecules in primary mouse astroglia via cell-to-cell contact.
MBP-primed T cells were mixed with either different concentrations of antibodies against the α4 (A – D) or β1 (E – H) chain of VLA-4 or control IgG and rocked gently for 1 h at room temperature. Cells were centrifuged, washed twice, and added to astroglia at a ratio of 0.5:1 T cell:glia. After 1 h of stimulation, culture dishes were shaken and washed to lower T cell concentration. (A & E), Adherent astroglia were incubated in serum-free media for 5 h and expression of iNOS was analyzed by semi-quantitative RT-PCR (A & E). After removal of T cells, adherent astroglia were incubated in serum-free media for 23 h and supernatants were used to assay nitrite (B & F), IL-1β (C & G) and IL-6 (D & H) as described in “Materials and Methods”. Plasma membranes of MBP-primed T cells were mixed with different concentrations of antibody against the β1 chain of VLA-4 or control IgG for 1h, washed and added to astroglia. After 6 h of incubation, expression of iNOS in astroglia was analyzed by semi-quantitative RT-PCR (I) and after 24 h of incubation, supernatents were used to assay nitrite (J) and IL-1β (K). Data are mean ± S.D. of three different experiments. a p < 0.001 vs. MBP-primed T cells only; b p < 0.05 vs. MBP-primed T cells only.
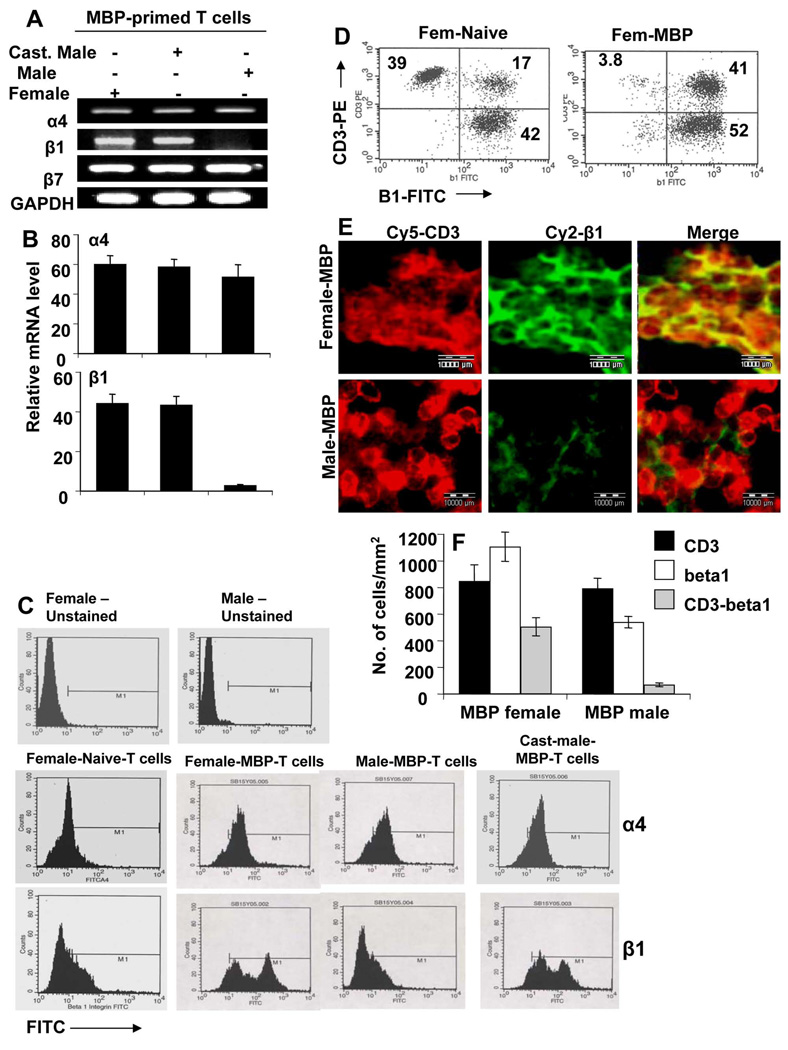
β1, but not α4, Integrin was Differentially Expressed in Female, Male and Castrated MBP-primed T cells of SJL/J Mice
Because α4 and β1 integrins play a vital role in T cell contact-mediated expression of proinflammatory molecules in glial cells and male MBP-primed T cells are incapable of inducing these molecules, we were prompted to investigate the expression pattern of these integrins in male and female mice. Apart from forming a heterodimer with β1, the α4 integrin forms heterodimers with other integrins like β7. Therefore, we decided to include β7 in the study as well. It is evident from semi-quantitative RT-PCR in figure 4A and real-time PCR in figure 4B that MBP-primed T cells of female, male and castrated male mice expressed α4 and β7 mRNAs. In contrast, MBP-primed T cells of female and castrated male, but not male, mice expressed β1 mRNA (Fig. 4A & 4B). Real-time PCR analysis shows that the mRNA expression of β1 in female and castrated male MBP-primed T cells was approximately 40-fold higher than that in male T cells (Fig. 4B).
Figure 4. Expression of α4 and β1 integrins in male, female and castrated male MBP-primed T cells and spleens.
MBP-primed T cells of female, male and castrated male mice were analyzed for the expression of α4, β1 and β7 integrins by semiquantitative RT-PCR (A). The mRNA expression of α4 and β1 in female, male and castrated male MBP-primed T cells were further analyzed by quantitative real-time PCR (B). Data are mean ± S.D. of three different experiments. Female, male and castrated male MBP-primed T cells were treated with appropriately diluted FITC-labeled antibodies against α4 or β1 or β1 and CD3 for 30 min followed by FACS analysis (C & D). Splenic cross sections of MBP-immunized female and male mice were double immunolabeled with antibodies against CD3 and β1 (E). Setting of the microscope was strictly unaltered during the whole study. Figures are representative of three independent experiments. (F) Cells positive for CD3, β1 or CD3 & β1 were counted in five splenic sections (3 images per slide) of each of three mice per group.
Because these integrins are surface molecules we confirmed our result by FACS analysis. Consistent to mRNA expression, we did not find any significant difference in the surface expression of α4 integrin among female, male and castrated male MBP-primed T cells (Fig. 4C; middle panel). However, the surface expression of β1 integrin was much higher in female and castrated male MBP-primed T cells than male MBP-primed T cells (Fig. 4C; bottom panel). We examined the pattern of surface expression of β1 integrin in CD3-positive cells. Dual FACS analysis for CD3 and β1 (Fig. 4D) clearly indicate significant increase in the expression of β1 in CD3-positive cells following immunization with MBP in female mice. It is also evident from our FACS data that apart from T cells, other nonadherent cells also express β1 at significant level (Fig. 4D). To further substantiate the fact, double-label immunofluorescence studies with anti-CD3 and anti-β1 antibodies in the splenic cross sections of MBP-immunized mice were performed and which also revealed dramatic decrease in expression of β1 in male compared to female, whereas there was no difference in CD3 expression (Fig. 4E). To quantitatively estimate the number of cells expressing CD3 and/or β1, the absolute numbers of cells were counted. Consistently, the results in figure 4F clearly indicate marked decrease in β1 producing cells in the spleen of male compared to female. Interestingly, apart from T cells which are CD3+, other splenic cells also expressed β1 integrin as evident from our results (Fig. 4F). The CD3-negative β1-producing splenic cells are likely to be macrophages which are the major antigen presenting cells in spleen. However, further studies are needed to confirm this fact. Taken together, our results suggest that inability of male MBP-primed T cells to induce the expression of iNOS, IL-1β and IL-6 in astroglia is probably due to the absence of β1 integrin in these cells and that the expression of this may be negatively regulated by male sex hormone.
Are Other Integrins Also Absent in Male MBP-primed T cells?
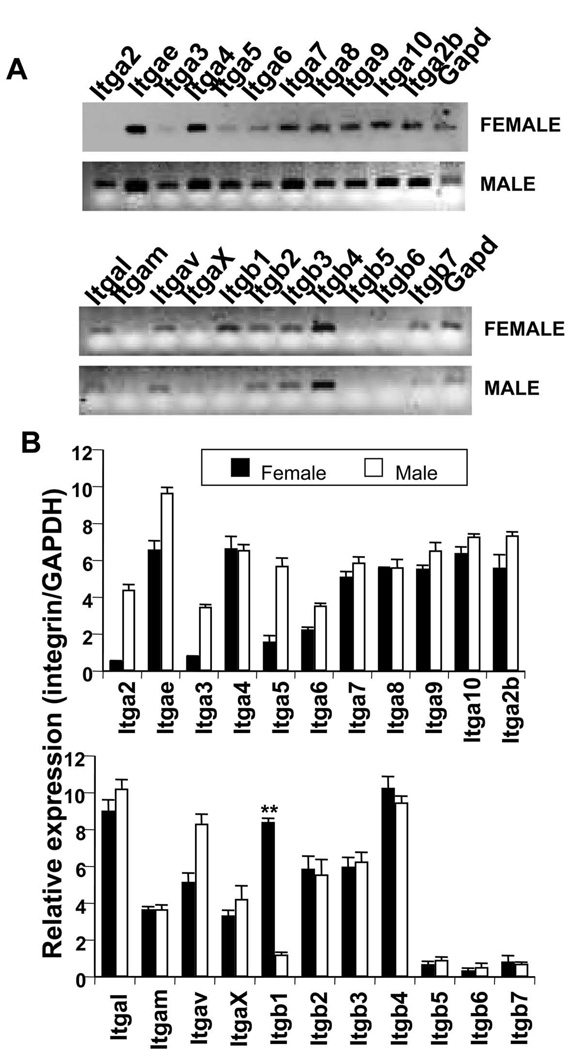
To examine whether β1 is the only integrin expressed differentially in female and male MBP-primed T cells, we analyzed gene expression profiles of mouse integrin α and β family of genes by RT-PCR gene-array analysis. We found that there was no significant difference in the expression of α4, α7, α8, α9, α10, α2b, αL, αM, αX, β2, β3, β4, β5, β6, and β7 between male and female MBP-primed T cells (Fig. 5A & 5B; Supplemental data). On the other hand, the expression of α2, α3, α5, and αV was higher in male MBP-primed T cells than female MBP-primed T cells (Fig. 5A & 5B; Supplemental data). Interestingly, β1 is the only integrin that was found to be almost missing from male MBP-primed T cells compared to female T cells (Fig. 5A & 5B). Because β1 integrin plays an important role in MBP-primed T cell contact-induced expression of proinflammatory molecules in astroglia (Fig. 4), our data strongly suggest that incapability of male T cells to induce proinflammatory molecules in astroglia is probably due to the absence of β1 integrin.
Figure 5. Gene array analysis of mouse integrin α and β gene families.
A, MBP-primed T cells of female and male mice were analyzed for α and β integrin gene families by Multigene-12™ PCR (SuperArray) as described under “Materials and Methods”. B, densitometric analysis was performed to show comparative expressions of α and β integrin genes between female and male MBP-primed T cells relative to GAPDH. Data are mean ± S.D. of three different experiments.
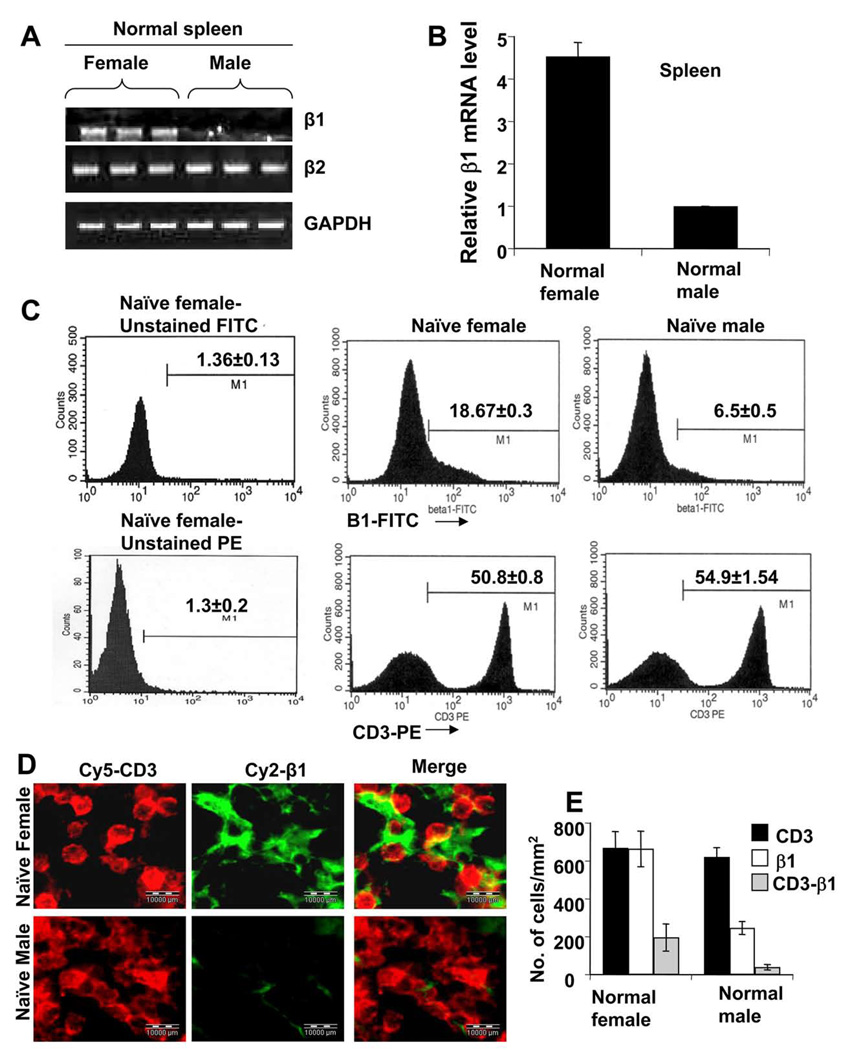
Is the Expression of β1 Integrin in Normal Young Female Mice Higher than Normal Young Male Mice?
We were further interested to see whether there was any difference in the expression of β1 integrin between normal young female and male SJL/J mice. Interestingly, both semi-quantitative and real-time RT-PCR analysis showed that spleen of naïve female SJL/J expressed significantly higher level of β1 compared to their male counterpart (Fig. 6A & 6B). The β1 integrin was dramatically low in male spleen (Fig. 6A & 6B). On the other hand, the expression of β2 integrin was same in both male and female MBP-primed T cells (Fig. 6A) suggesting the specificity of our observation. Because integrins are surface molecules, to strengthen our finding, protein level of the β1 were analyzed by FACS. Consistently, we observed marked reduction in expression of β1 in naïve splenocytes of male compared to female, but there was no difference in expression of CD3 (Fig. 6C).
Figure 6. Expression of β1 integrin in spleens of naïve young female and male mice.
RNA isolated from spleen of naïve young female and male SJL/J mice (4 to 6 week old) was analyzed for the mRNA expression of β1 and β2 integrins by semiquantitative RT-PCR (A). The mRNA expression of β1 integrin was also confirmed by quantitative Realtime PCR (B). Data are mean ± S.D. of three different experiments. a p < 0.001 vs. male. Naïve female and male splenocytes after isolation, were treated with appropriately diluted FITC-labeled antibodies against β1 or PE-labeled antibodies against CD3 for 30 min followed by FACS analysis (C). Splenic cross sections of naïve young female and male mice were double immunolabeled with antibodies against CD3 and β1 (D). Setting of the microscope was strictly unaltered during the whole study. Figures are representative of three independent experiments. (E) Cells positive for CD3, β1 or CD3 & β1 were counted in five splenic sections (3 images per slide) of each of three mice per group.
To further substantiate these finding, dual immunohistochemical studies for CD3 and β1 were performed on splenic sections of naïve mouse. Here also it showed dramatic decrease in expression of β1 in the spleen of naïve young male in comparison to female without any alteration of expression of CD3 (Fig. 6D). The quantitative estimation of CD3 and/or β1-positive cells further conformed to our immunohistochemical studies (Fig. 6E). Interestingly, majority of the β1-positive cells were found to be CD3-negative suggesting that in naïve spleen, T cells do not express significant amount of β1. Therefore, it is likely that majority of the β1-producing cells in naïve spleen are macrophages, which are the major APCs, but it requires further studies to establish this fact. However, irrespective of cell type, the level of β1 is abruptly low in naïve male spleen, which clearly indicates that male-specific reduction in β1 expression is not only limited to T cells but also includes other splenic cells expressing the integrin β1. Taken together, these observations suggest that the difference in expression of β1 between male and female SJL/J mice is not a result of MBP immunization; rather it exists normally, and therefore, could be described as an intrinsic sex-related phenomenon.
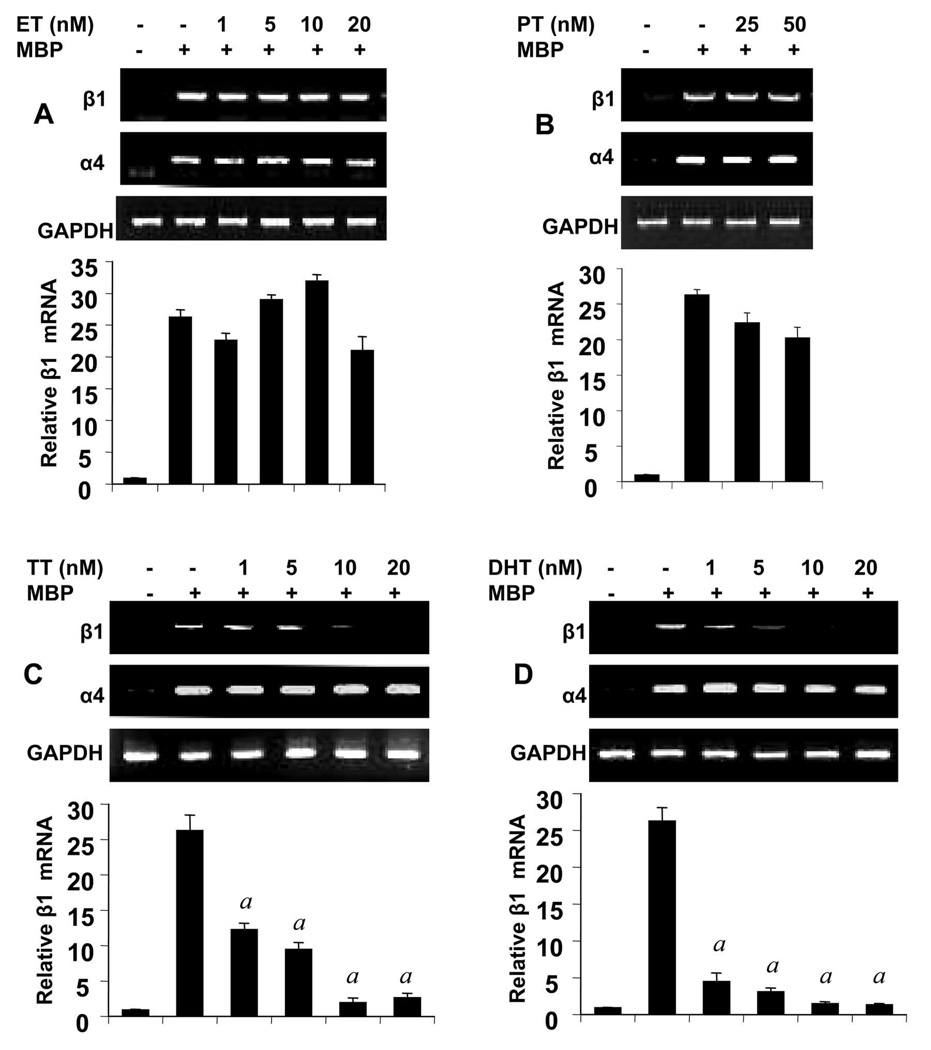
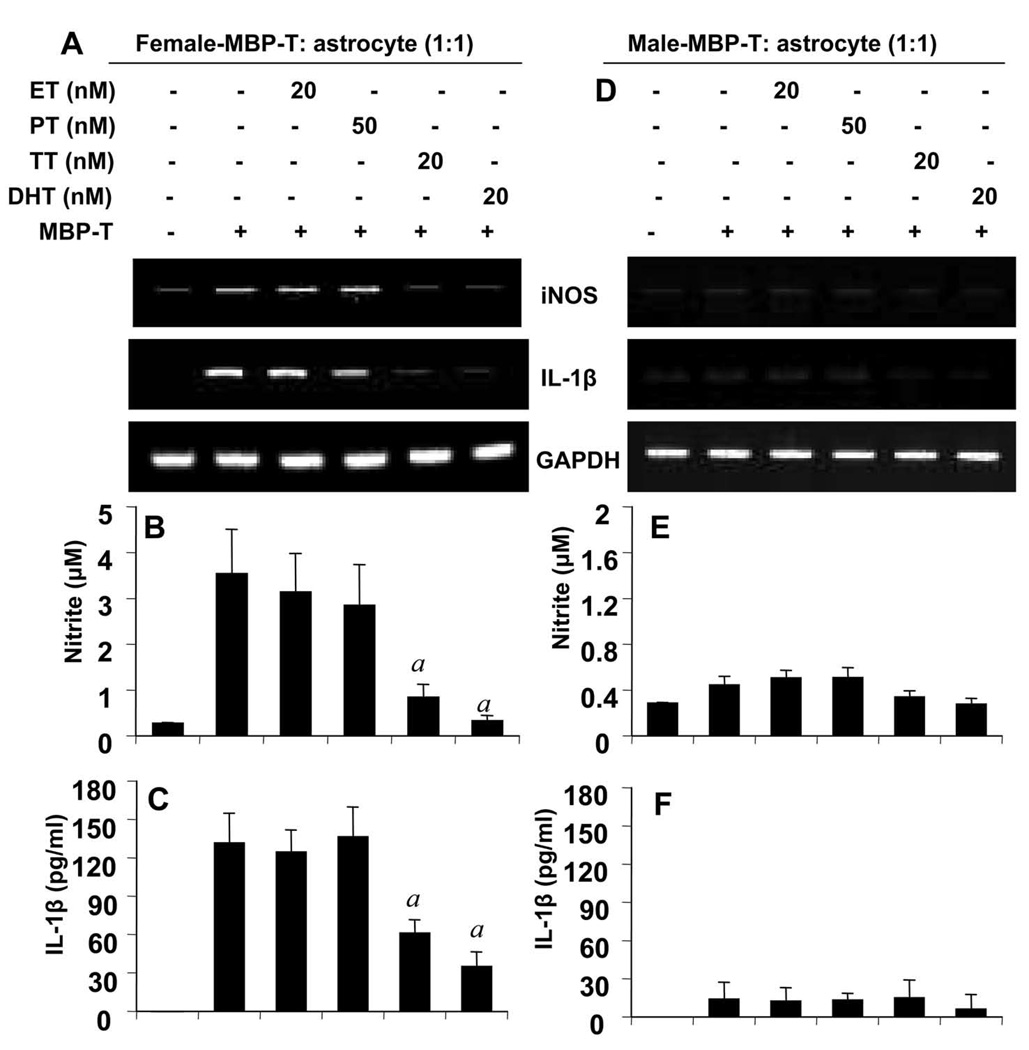
Testosterone (TT) and dihydrotestosterone (DHT), but not Estrogen (ET) and Progesterone (PT), Selectively Inhibited the Expression of β1 in Female MBP-primed T cells in a Dose-dependent Manner
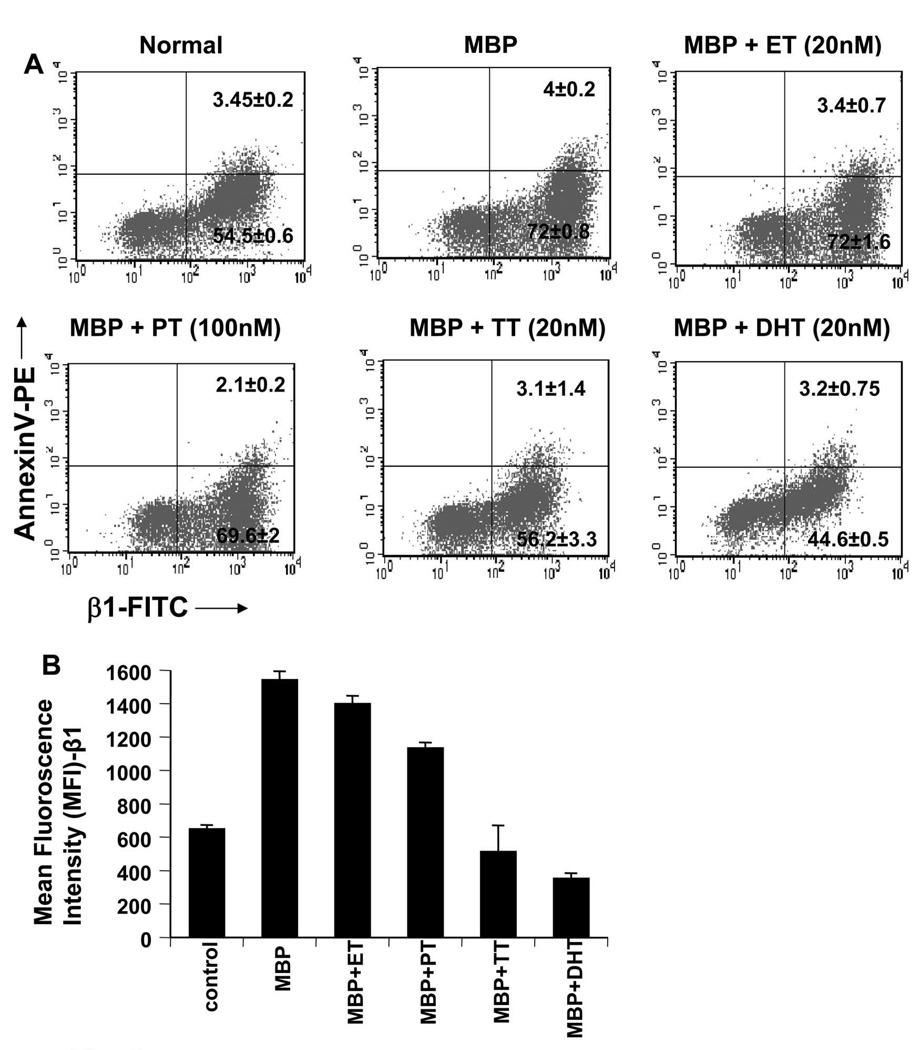
Because castration of male SJL/J resulted in marked increase in the expression of β1 at a level same as female MBP-T cells, we investigated whether androgens had any effect on the expression of this integrin. Female MBP-primed T cells were treated with physiologic doses of male and female sex hormones during MBP-priming. As evident from RT-PCR and quantitative real-time PCR analysis, both TT (Fig. 7C) and DHT (Fig. 7D) dose-dependently inhibited the expression of β1 integrin in female MBP-primed T cells. However, at the same condition, TT and DHT had no effect on the expression of α4 integrin, the partner of β1 in VLA4 (Fig 7C & 7D). This was consistent with our observation that castration of male did not alter the expression level of α4 integrin (Fig. 4A). These results suggest that the inhibitory effect of male sex hormones is specific for β1 integrin of VLA4. On the other hand, as evident from figure 7A and 7B, female-specific hormones (ET and PT) at physiologic doses had minimal or no effect on the expression of either β1 or α4 integrin. To confirm the results further, we also performed FACS analysis. Two-color FACS analysis using PE labeled Annexin-V and FITC-labeled β1 revealed that there was no significant apoptotic cell death of T cells after MBP and/or hormone treatments (Fig. 8A, upper right quadrants). The FACS analysis further showed that, both TT and DHT, but not estrogen or progesterone, significantly reduced the proportion of β1 integrin-positive cells in the MBP-primed T cells (Fig. 8A, lower right quadrants). Although, there was substantial reduction in β1+ MBP-primed T cells by testosterone or DHT treatment, still approximately around 50% of MBP-primed T cells treated with testosterone or DHT were found to be β1+. On the contrary, mRNA level of β1 was almost completely absent in testosterone or DHT-treated female MBP-primed T cells (Fig. 7C & D). This apparent discrepancy could be explained by the fact that half-life of the integrins which are surface molecules, are usually relatively longer than other molecules. Therefore, although mRNA expression was inhibited completely, protein level was still there.
Figure 7. Effect of estrogen (ET), progesterone (PT), testosterone (TT), and dihydrotestosterone (DHT) on the expression of α4 and β1 integrins in female MBP-primed T cells.
MBP-primed T cells treated with different concentrations of ET (A), PT (B), TT (C), or DHT (D) for 72 h during MBP priming were analyzed for the mRNA expression of α4 and β1 integrins by semi-quantitative RT-PCR (upper panels) and realtime PCR (lower panels). Data are mean ± S.D. of three different experiments. a p < 0.001 vs. MBP only.
Figure 8. Effect of estrogen (ET), progesterone (PT), testosterone (TT), and dihydrotestosterone (DHT) on the surface expression of β1 integrin in female MBP-primed T cells.
Female MBP-primed T cells treated with ET, PT, TT, and DHT for 72 h during MBP priming were incubated with appropriately diluted FITC-labeled anti-VLA-4 β1 and PE-labeled annexin-V for 1 h followed by two-color FACS analysis. Figures represent three independent experiments (A). Mean fluorescence intensity of β1 in FITC-positive cells were calculated by using CellQuest software (B). Data are ±S.D. of three independent experiments.
To further confirm whether there was significant down-regulation of integrin β1 in MBP-primed T cells following hormone treatment, we also analyzed the expression level of β1 per cell by calculating MFI of β1+ cells. Expectedly we found that, both testosterone and DHT, but not estrogen or progesterone, markedly down-regulated the expression of β1 in MBP-primed T cells (Fig. 8B). Interestingly, DHT appeared to have stronger effect than TT on the expression β1 (Fig. 7D, Fig. 8A & B). This could be because of aromatase activity which is capable of converting some TT to ET while DHT, the active metabolite of TT, remains unaffected. These findings suggest that male- but not female-specific hormones are capable of down-regulating the β1 subunit of VLA-4 integrin at physiological doses.
TT and DHT, but not ET and PT, Inhibited the Ability of Female MBP-primed T Cells to Induce Contact-mediated Expression of proinflammatory molecules in Mouse Primary Astroglia
Because of our findings that testosterone and DHT treatment of female MBP-primed T cells resulted in the inhibition of β1 and that β1 was found to be necessary for T cell contact-mediated induction of proinflammatory molecules in mouse primary astroglia, we were interested to examine whether androgens down-regulated this contact activity of MBP-primed T cells. Female MBP-primed T cells were treated with TT, DHT, ET, and PT followed by addition of hormone-treated T cells to astroglia in direct contact. Consistent to the inhibition of β1 integrin, both TT and DHT suppressed the ability of female MBP-primed T cells to induce contact-mediated expression of proinflammatory molecules (iNOS and IL-1β) (Fig. 9A) and production of NO (Fig. 9B) and IL-1β (Fig. 9C) in astroglia. Expectedly, female-specific hormones (ET and PT) were unable to inhibit this contact activity of female MBP-primed T cells (Fig. 9A, 9B and 9C). In parallel experiments, we also examined whether these male and female sex hormones had any effect on the missing proinflammatory contact activity of male MBP-primed T cells. As evident from figure 9D, 9E and 9F, all four sex steroids had no effect on the missing contact activity in male MBP-primed T cells.
Figure 9. Effect of estrogen (ET), progesterone (PT), testosterone (TT), and dihydrotestosterone (DHT) on the ability of female and male MBP-primed T cells to induce contact-mediated expression of proinflammatory molecules in primary mouse astroglia.
Female (A – C) and male (D – F) MBP-primed T cells treated with respective concentrations of ET, PT, TT, and DHT for 72 h during MBP priming were added to astroglia at a ratio of 1:1 T cell:astroglia. After 1 h of stimulation, culture dishes were shaken and washed to lower T cell concentration. Then adherent astroglia were incubated in serum-free media for 5 h and the expression of iNOS mRNA was analyzed by semiquantitative RT-PCR (A & D). Adherent astroglia were incubated in serum-free media for 23 h and supernatants were used to assay nitrite (B & E) and IL-1β (C & F). Data are mean ± S.D. of three different experiments. a p < 0.001 vs. MBP-primed T cells only.
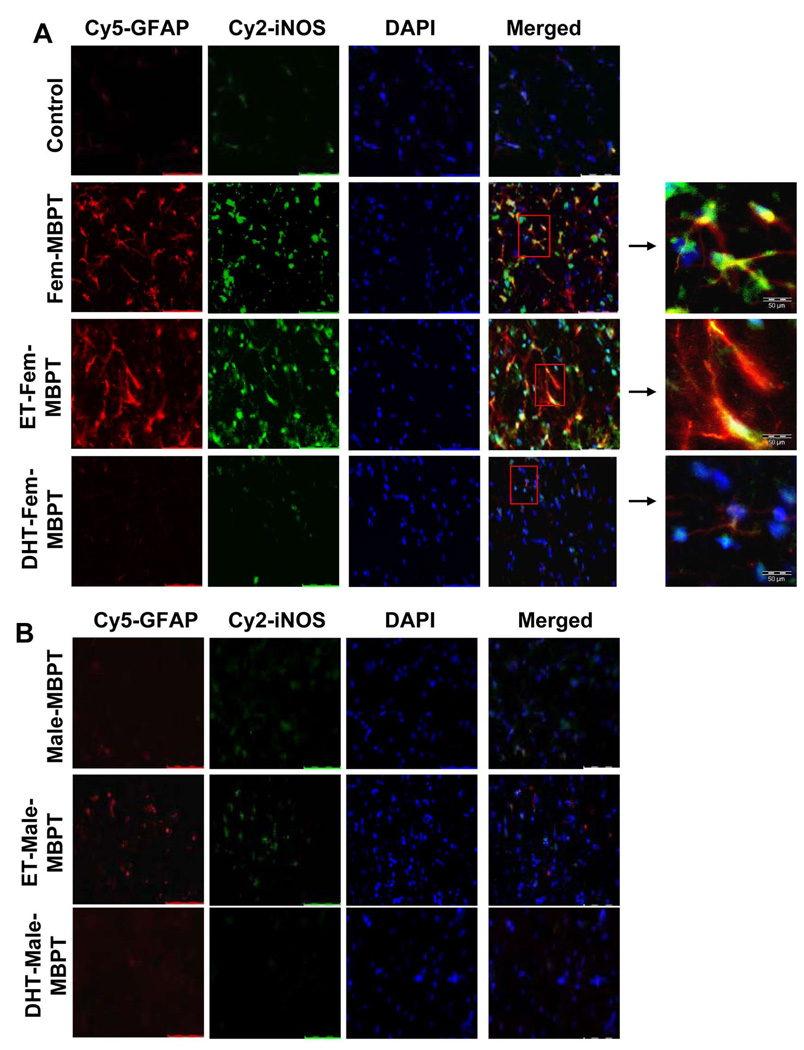
DHT, but not ET, inhibited the ability of female MBP-primed T cells to induce iNOS in vivo in the cerebellum of adoptively-transferred mice
To confirm our findings in vivo, we examined the expression of iNOS and GFAP, the marker of astroglial activation, in the cerebellum of female SJL/J mice that received adoptive transfer of hormone-treated or untreated female or male MBP-primed T cells. Consistent with our in vitro results, mice transferred with female MBP-primed T cells or ET-treated female MBP-primed T cells showed significant increase in the level of iNOS in astroglial cells compared to control (Fig. 10A; 2nd and 3rd rows). Parallel increase in GFAP in iNOS-producing astroglia indicates astroglial activation, which is consistent with our previous studies (24). Expectedly, DHT treatment of MBP-primed T cells markedly reduced the level iNOS and GFAP in the cerebellum, thereby further confirming the consequence of hormonal regulation of β1 integrin in T cells under in vivo condition (Fig. 10A; bottom row). Similar to our in vitro results, mice receiving male MBP-primed T cells did not show any significant level of iNOS (Fig. 10B; 1st row) and hormone treatment of male MBP-primed T cells almost had no effect (Fig 10B).
Figure 10. Effect of ET and DHT on the ability of female and male MBP-primed T cells to induce expression of iNOS in the cerebellum of adoptively-transferred mice.
Female (A) and male (B) MBP-primed T cells, treated with appropriate concentrations of ET or DHT for 96 h during MBP priming, were adoptively transferred to female SJL/L mice. On day 5 post immunization, cerebellar sections were double-immunolabelled with antibodies against iNOS and GFAP. Setting of the microscope was strictly unaltered during the whole study. Figures are representative of three independent experiments.
DISCUSSION
T cell-mediated autoimmune response is believed to cause damage in the CNS of MS patients. Because lymph node and spleen, the two primary activation sites T cells, express myelin basic protein mRNA and protein in rat, mouse and human (36), it is widely believed that neuroantigen-specific autoimmune T cells are activated at those sites and thereby infiltrate into CNS after crossing BBB. In the CNS microenvironment, these T cells recognize their antigens, interact with resident glial cells and subsequent glial activation trigger a broad-spectrum inflammatory cascade which ultimately results in oligodendrocyte death and demyelination. It may be likely that as females are more susceptible to MS than males, the neuroantigen-specific T cells do more severe CNS damage in female than in male. However, the exact molecular mechanism behind the sexual dimorphism of CNS neurodegeneration in MS is unknown.
We have previously reported that MBP-primed T cell contact-mediated activation of microglia is gender sensitive (22). Because astroglia constitute majority of resident glial cells, astroglial activation could also play a vital role in the pathogenesis of MS and EAE. In this manuscript, we have presented substantial evidences which support that MBP-primed T cell contact-mediated astroglial activation is also gender-sensitive. First, female MBP-primed T cells dose-dependently induced the expression of iNOS and proinflammatory cytokines (IL-1β & IL-6) as well as the production of these proinflammatory molecules in primary mouse astroglia. Either T cell in direct contact or T cell membrane was capable of inducing proinflammatory molecules in astroglia. On the other hand, T cells placed on inserts and supernatants of T cells were unable to induce the same proinflammatory molecules in astroglia suggesting that this induction was purely because of direct contact between T cells and astroglia. Second, female and castrated-male MBP-primed T cells, but not male MBP-primed T cells, were able to induce the expression of proinflammatory molecules (iNOS, IL-1β & IL-6) in astroglia, clearly suggesting the gender-specificity of astroglial activation driven by MBP-primed T cell contacts.
We next investigated the underlying mechanism behind the inability of male MBP-primed T cells to activate astroglia via contact. Because VLA-4, according to our previous report (21), plays an important role in contact-mediated induction proinflammatory molecules in microglia and as VLA-4 is a heterodimer of α4 and β1, we examined the role of α4 and β1 integrins in contact-mediated induction of proinflammatory molecules in astroglia. Impairing the function of either α4 or β1 integrin of VLA-4 of female MBP-primed T cells significantly inhibited their ability to induce the expression of proinflammatory molecules in mouse astroglia, suggesting an essential role of each of the subunits of VLA-4 integrin in contact-mediated activation of astroglia. However, how VLA-4 leads to astroglial activation, is not understood yet. The contact molecule for VLA-4 in astroglia is probably the vascular cell adhesion molecule-1 (VCAM-1). Activated T cells secrete various proinflammatory molecules such as IFNγ and TNFα and these molecules are capable of upregulating VCAM-1 in astrocytes (37). Blocking of VLA-4 or VCAM-1 have been shown to prevent T cell adhesion to astrocytes, suggesting that VLA-4-VCAM-1 interaction is necessary for T cell adhesion to astrocytes (37). Moreover, induction of VCAM-1 has been found to be associated with astroglial activation in the spinal cord of EAE, an model of MS (38). However, the downstream signaling events leading to astroglial activation, are yet to be investigated. As we have previously reported that VLA-4-mediated microglial activation by T cell contact involves activation of C/EBPβ (21, 22), it can be speculated that this molecule may also be involved in astroglial activation by T cell contact.
Requirement of VLA-4 subunits in T cell contact-mediated induction of proinflammatory molecules in astrocytes, prompted us to examine whether there was any gender biasness in the expression of individual subunits of VLA-4 in T cells. Surprisingly, the expression of only β1 integrin, but not α4 and others, was found significantly less in male MBP-primed T cells compared to females. However, after castration, castrated male MBP-primed T cells expressed β1 at a level comparable to female MBP-primed T cells. These studies strongly suggest that inability of male MBP-primed T cells to activate astroglia is attributed by diminished level of β1 subunit of VLA-4.
We next asked why the male SJL/J has defective β1. Comparable level of β1 in castrated male-MBP-primed T cells suggested that sex hormones might play an important role in the regulation β1. Sex steroids are important modulators of disease processes of MS and EAE. Sex hormones can have both immunomodulatory and neuroprotective effect on EAE (39–42). Both testosterone and estrogen have been found to be protective in EAE (40, 42, 43). The immunomodulatory effect of male sex hormones on EAE is mostly exerted through reduction of Th1 cytokines. However, immunomodulatory and neuroprotective effects of estrogen are specifically mediated by ERα, but not ERβ (44). Therefore it is likely that distribution of ER as well as the estrogen level may play critical role in determining the beneficial role of estrogen in MS and EAE. It is probably the higher level of estrogen than the normal physiological level that protects MS and other autoimmune diseases. Probably because of this reason, MS patients as well as patients of other autoimmune diseases experience reduced clinical symptoms during pregnancy when estrogen level goes up (45, 46).
In our current study we found that testosterone and DHT dose-dependently down-regulated expression of β1, but not α4, of female MBP-primed T cells whereas estrogen and progesterone had no significant effect. Effect of DHT was relatively stronger than testosterone. It is probably because of aromatase activity that could convert some testosterone, but not DHT, to estrogen (47). We further demonstrated that, contact-mediated induction of proinflammatory molecules in astroglia was significantly inhibited by testosterone or DHT-treated female MBP-primed T cells but the same T cells when treated with estrogen or progesterone were incapable of inhibiting the induction of proinflammatory molecules in astroglia. Our in vivo results further substantiated this finding where we noticed that mice adoptively transferred with DHT-treated, but not ET-treated, female MBP-primed T cells showed dramatically low level of iNOS and GFAP, the marker protein of astroglial activation. Therefore, inability of female-derived MBP-primed T cells treated with testosterone or DHT to express specifically β1 and subsequently, the inability of those cells to produce proinflammatory molecules in mouse primary astroglia via contact clearly suggest that specifically the β1 subunit of VLA-4 is negatively regulated by male sex hormones and thereby these hormones inhibit T cell contact-mediated astroglial activation. Thus our results explore a novel mechanistic aspect of protective role of male sex hormone that emphasizes the importance of T cell contact-mediated CNS inflammation in the gender susceptibility of EAE.
In summary, we have delineated a possible mechanism of sexual dimorphism in MS. Because even the naïve young male mice did not express β1, this integrin may turn out to be a susceptibility marker for MS and thus it may be considered as a possible gender-specific factor for increased incidence of MS in females. Our study also reveals a possible new direction for MS therapy. Tysabri, as a new avatar of antegrin, which is a functional blocking antibody against α4 integrin of VLA4, is rocking the headlines as a new treatment for MS. However, Tysabri increases the risk of progressive multifocal leukoencephalopathy (PML), an opportunistic viral infection of the brain that usually leads to death or severe disability. On the other hand, if our mouse results are extrapolated to human, young males should have lower level of β1. However, despite the deficiency of β1, young males do fine because other integrins are present in male T cells at a comparable level or at a level higher than female T cells. According to our unpublished observation, like female MBP-primed T cells, the male MBP-primed T cells were also capable of infiltrating into the CNS. This observation suggests that function of VLA-4 was compensated by some other molecule(s) which facilitated the extravasation male T cells as efficiently as that of females. Although β1 null mice are embryonic lethal (48–50); this is because the integrin β1 is critically required for embryogenesis. On the other hand, our experimental findings suggest that absence or low amount of β1 in male is probably due to the regulation by male-specific sex hormones. As sex hormones can play a role only during puberty and onwards, it is likely that during embryogenesis, the level of β1 integrin in male is normal. Therefore, developing functional blocking antibodies against β1 may provide a safer handle than ‘Tysabri’ to contain MS and other autoimmune disorders.
Supplementary Material
Acknowledgments
This study was supported by grants from NIH (NS39940 and NS48923). The University of Rush Flow facility was supported in part by the James. B. Pendleton Charitable Trust.
REFERENCES
- 1.Duquette P, Pleines J, Girard M, Charest L, Senecal-Quevillon M, Masse C. The increased susceptibility of women to multiple sclerosis. The Canadian journal of neurological sciences. 1992;19:466–471. [PubMed] [Google Scholar]
- 2.Voskuhl RR, Palaszynski K. Sex hormones in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Neuroscientist. 2001;7:258–270. doi: 10.1177/107385840100700310. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clinical immunology and immunopathology. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 4.Voskuhl RR, Pitchekian-Halabi H, MacKenzie-Graham A, McFarland HF, Raine CS. Gender differences in autoimmune demyelination in the mouse: implications for multiple sclerosis. Annals of neurology. 1996;39:724–733. doi: 10.1002/ana.410390608. [DOI] [PubMed] [Google Scholar]
- 5.Roubinian JR, Talal N, Greenspan JS, Goodman JR, Siiteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. The Journal of experimental medicine. 1978;147:1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato EH, Ariga H, Sullivan DA. Impact of androgen therapy in Sjogren's syndrome: hormonal influence on lymphocyte populations and Ia expression in lacrimal glands of MRL/Mp-lpr/lpr mice. Investigative ophthalmology & visual science. 1992;33:2537–2545. [PubMed] [Google Scholar]
- 7.Staykova MA, Fordham SA, Bartell GJ, Cowden WB, Willenborg DO. Nitric oxide contributes to the resistance of young SJL/J mice to experimental autoimmune encephalomyelitis. Journal of neuroimmunology. 2006;176:1–8. doi: 10.1016/j.jneuroim.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Palaszynski KM, Loo KK, Ashouri JF, Liu HB, Voskuhl RR. Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Journal of neuroimmunology. 2004;146:144–152. doi: 10.1016/j.jneuroim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. Journal of molecular medicine (Berlin, Germany) 1997;75:165–173. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- 10.Du C, Khalil MW, Sriram S. Administration of dehydroepiandrosterone suppresses experimental allergic encephalomyelitis in SJL/J mice. J Immunol. 2001;167:7094–7101. doi: 10.4049/jimmunol.167.12.7094. [DOI] [PubMed] [Google Scholar]
- 11.Parkinson JF, Mitrovic B, Merrill JE. The role of nitric oxide in multiple sclerosis. Journal of molecular medicine (Berlin, Germany) 1997;75:174–186. doi: 10.1007/s001090050102. [DOI] [PubMed] [Google Scholar]
- 12.Pahan K, Schmid M. Activation of nuclear factor-kB in the spinal cord of experimental allergic encephalomyelitis. Neuroscience letters. 2000;287:17–20. doi: 10.1016/s0304-3940(00)01167-8. [DOI] [PubMed] [Google Scholar]
- 13.Bruck W, Stadelmann C. Inflammation and degeneration in multiple sclerosis. Neurol Sci. 2003;24 Suppl 5:S265–S267. doi: 10.1007/s10072-003-0170-7. [DOI] [PubMed] [Google Scholar]
- 14.Bo L, Dawson TM, Wesselingh S, Mork S, Choi S, Kong PA, Hanley D, Trapp BD. Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Annals of neurology. 1994;36:778–786. doi: 10.1002/ana.410360515. [DOI] [PubMed] [Google Scholar]
- 15.Bagasra O, Michaels FH, Zheng YM, Bobroski LE, Spitsin SV, Fu ZF, Tawadros R, Koprowski H. Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:12041–12045. doi: 10.1073/pnas.92.26.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooper DC, Bagasra O, Marini JC, Zborek A, Ohnishi ST, Kean R, Champion JM, Sarker AB, Bobroski L, Farber JL, Akaike T, Maeda H, Koprowski H. Prevention of experimental allergic encephalomyelitis by targeting nitric oxide and peroxynitrite: implications for the treatment of multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2528–2533. doi: 10.1073/pnas.94.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharief MK, Hentges R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. The New England journal of medicine. 1991;325:467–472. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- 18.Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol. 1998;161:6480–6486. [PubMed] [Google Scholar]
- 19.Maimone D, Gregory S, Arnason BG, Reder AT. Cytokine levels in the cerebrospinal fluid and serum of patients with multiple sclerosis. Journal of neuroimmunology. 1991;32:67–74. doi: 10.1016/0165-5728(91)90073-g. [DOI] [PubMed] [Google Scholar]
- 20.Ruddle NH, Bergman CM, McGrath KM, Lingenheld EG, Grunnet ML, Padula SJ, Clark RB. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. The Journal of experimental medicine. 1990;172:1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dasgupta S, Jana M, Liu X, Pahan K. Role of very-late antigen-4 (VLA-4) in myelin basic protein-primed T cell contact-induced expression of proinflammatory cytokines in microglial cells. The Journal of biological chemistry. 2003;278:22424–22431. doi: 10.1074/jbc.M301789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dasgupta S, Jana M, Liu X, Pahan K. Myelin basic protein-primed T cells of female but not male mice induce nitric-oxide synthase and proinflammatory cytokines in microglia: implications for gender bias in multiple sclerosis. The Journal of biological chemistry. 2005;280:32609–32617. doi: 10.1074/jbc.M500299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malmestrom C, Haghighi S, Rosengren L, Andersen O, Lycke J. Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology. 2003;61:1720–1725. doi: 10.1212/01.wnl.0000098880.19793.b6. [DOI] [PubMed] [Google Scholar]
- 24.Brahmachari S, Fung YK, Pahan K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci. 2006;26:4930–4939. doi: 10.1523/JNEUROSCI.5480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herx LM, Yong VW. Interleukin-1 beta is required for the early evolution of reactive astrogliosis following CNS lesion. Journal of neuropathology and experimental neurology. 2001;60:961–971. doi: 10.1093/jnen/60.10.961. [DOI] [PubMed] [Google Scholar]
- 26.Van Wagoner NJ, Benveniste EN. Interleukin-6 expression and regulation in astrocytes. Journal of neuroimmunology. 1999;100:124–139. doi: 10.1016/s0165-5728(99)00187-3. [DOI] [PubMed] [Google Scholar]
- 27.Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pahan K, Liu X, McKinney MJ, Wood C, Sheikh FG, Raymond JR. Expression of a dominant-negative mutant of p21(ras) inhibits induction of nitric oxide synthase and activation of nuclear factor-kappaB in primary astrocytes. Journal of neurochemistry. 2000;74:2288–2295. doi: 10.1046/j.1471-4159.2000.0742288.x. [DOI] [PubMed] [Google Scholar]
- 29.Jana M, Liu X, Koka S, Ghosh S, Petro TM, Pahan K. Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. The Journal of biological chemistry. 2001;276:44527–44533. doi: 10.1074/jbc.M106771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pahan K, Sheikh FG, Liu X, Hilger S, McKinney M, Petro TM. Induction of nitric-oxide synthase and activation of NF-kappaB by interleukin-12 p40 in microglial cells. The Journal of biological chemistry. 2001;276:7899–7905. doi: 10.1074/jbc.M008262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jana M, Dasgupta S, Liu X, Pahan K. Regulation of tumor necrosis factor-alpha expression by CD40 ligation in BV-2 microglial cells. Journal of neurochemistry. 2002;80:197–206. doi: 10.1046/j.0022-3042.2001.00691.x. [DOI] [PubMed] [Google Scholar]
- 32.Roy A, Fung YK, Liu X, Pahan K. Up-regulation of microglial CD11b expression by nitric oxide. The Journal of biological chemistry. 2006;281:14971–14980. doi: 10.1074/jbc.M600236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brahmachari S, Pahan K. Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis. J Immunol. 2007;179:275–283. doi: 10.4049/jimmunol.179.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dasgupta S, Jana M, Liu X, Pahan K. Myelin basic protein-primed T cells induce nitric oxide synthase in microglial cells. Implications for multiple sclerosis. The Journal of biological chemistry. 2002;277:39327–39333. doi: 10.1074/jbc.M111841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snow AL, Lambert SL, Natkunam Y, Esquivel CO, Krams SM, Martinez OM. EBV can protect latently infected B cell lymphomas from death receptor-induced apoptosis. J Immunol. 2006;177:3283–3293. doi: 10.4049/jimmunol.177.5.3283. [DOI] [PubMed] [Google Scholar]
- 36.Liu H, MacKenzie-Graham AJ, Palaszynski K, Liva S, Voskuhl RR. "Classic" myelin basic proteins are expressed in lymphoid tissue macrophages. Journal of neuroimmunology. 2001;116:83–93. doi: 10.1016/s0165-5728(01)00284-3. [DOI] [PubMed] [Google Scholar]
- 37.Rosenman SJ, Shrikant P, Dubb L, Benveniste EN, Ransohoff RM. Cytokine-induced expression of vascular cell adhesion molecule-1 (VCAM-1) by astrocytes and astrocytoma cell lines. J Immunol. 1995;154:1888–1899. [PubMed] [Google Scholar]
- 38.Archambault AS, Sim J, McCandless EE, Klein RS, Russell JH. Region-specific regulation of inflammation and pathogenesis in experimental autoimmune encephalomyelitis. Journal of neuroimmunology. 2006;181:122–132. doi: 10.1016/j.jneuroim.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Gold SM, Voskuhl RR. Estrogen and testosterone therapies in multiple sclerosis. Progress in brain research. 2009;175:239–251. doi: 10.1016/S0079-6123(09)17516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalal M, Kim S, Voskuhl RR. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol. 1997;159:3–6. [PubMed] [Google Scholar]
- 41.Bebo BF, Jr, Schuster JC, Vandenbark AA, Offner H. Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J Immunol. 1999;162:35–40. [PubMed] [Google Scholar]
- 42.Subramanian S, Matejuk A, Zamora A, Vandenbark AA, Offner H. Oral feeding with ethinyl estradiol suppresses and treats experimental autoimmune encephalomyelitis in SJL mice and inhibits the recruitment of inflammatory cells into the central nervous system. J Immunol. 2003;170:1548–1555. doi: 10.4049/jimmunol.170.3.1548. [DOI] [PubMed] [Google Scholar]
- 43.Palaszynski KM, Liu H, Loo KK, Voskuhl RR. Estriol treatment ameliorates disease in males with experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Journal of neuroimmunology. 2004;149:84–89. doi: 10.1016/j.jneuroim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 44.Offner H, Polanczyk M. A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis. Annals of the New York Academy of Sciences. 2006;1089:343–372. doi: 10.1196/annals.1386.021. [DOI] [PubMed] [Google Scholar]
- 45.Damek DM, Shuster EA. Pregnancy and multiple sclerosis. Mayo Clinic proceedings. 1997;72:977–989. doi: 10.1016/S0025-6196(11)63371-5. [DOI] [PubMed] [Google Scholar]
- 46.Runmarker B, Andersen O. Pregnancy is associated with a lower risk of onset and a better prognosis in multiple sclerosis. Brain. 1995;118(Pt 1):253–261. doi: 10.1093/brain/118.1.253. [DOI] [PubMed] [Google Scholar]
- 47.Zheng R, Samy TS, Schneider CP, Rue LW, 3rd, Bland KI, Chaudry IH. Decreased 5alpha-dihydrotestosterone catabolism suppresses T lymphocyte functions in males after trauma-hemorrhage. American journal of physiology. 2002;282:C1332–C1338. doi: 10.1152/ajpcell.00560.2001. [DOI] [PubMed] [Google Scholar]
- 48.Brakebusch C, Fassler R. beta 1 integrin function in vivo: adhesion, migration and more. Cancer metastasis reviews. 2005;24:403–411. doi: 10.1007/s10555-005-5132-5. [DOI] [PubMed] [Google Scholar]
- 49.Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes & development. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 50.Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes & development. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.