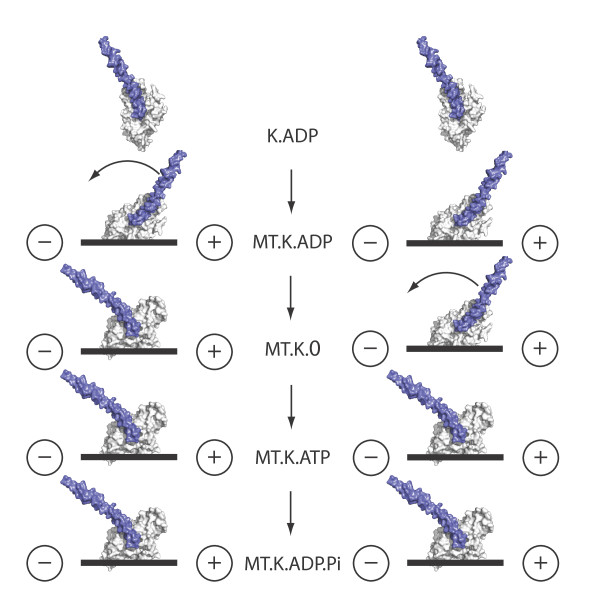
Figure 2.
Competing models for coupling the kinesin-14 lever scheme to ATP turnover. In one scheme (left), lever motion is coupled to ADP release. In the other (right), it is coupled to ATP binding. K.ADP, kinesin with ADP bound in the active site; MT.K.ADP, kinesin with bound ADP in contact with microtubule (MT); MT.K.0, complex of microtubule and kinesin after ADP release; MT.K.ATP, complex of microtubule and kinesin with bound ATP; MT.K.ADP.Pi, hydrolysis of ATP generates kinesin with ADP and Pi bound. The - and + signs indicate the minus and plus ends of the microtubule.