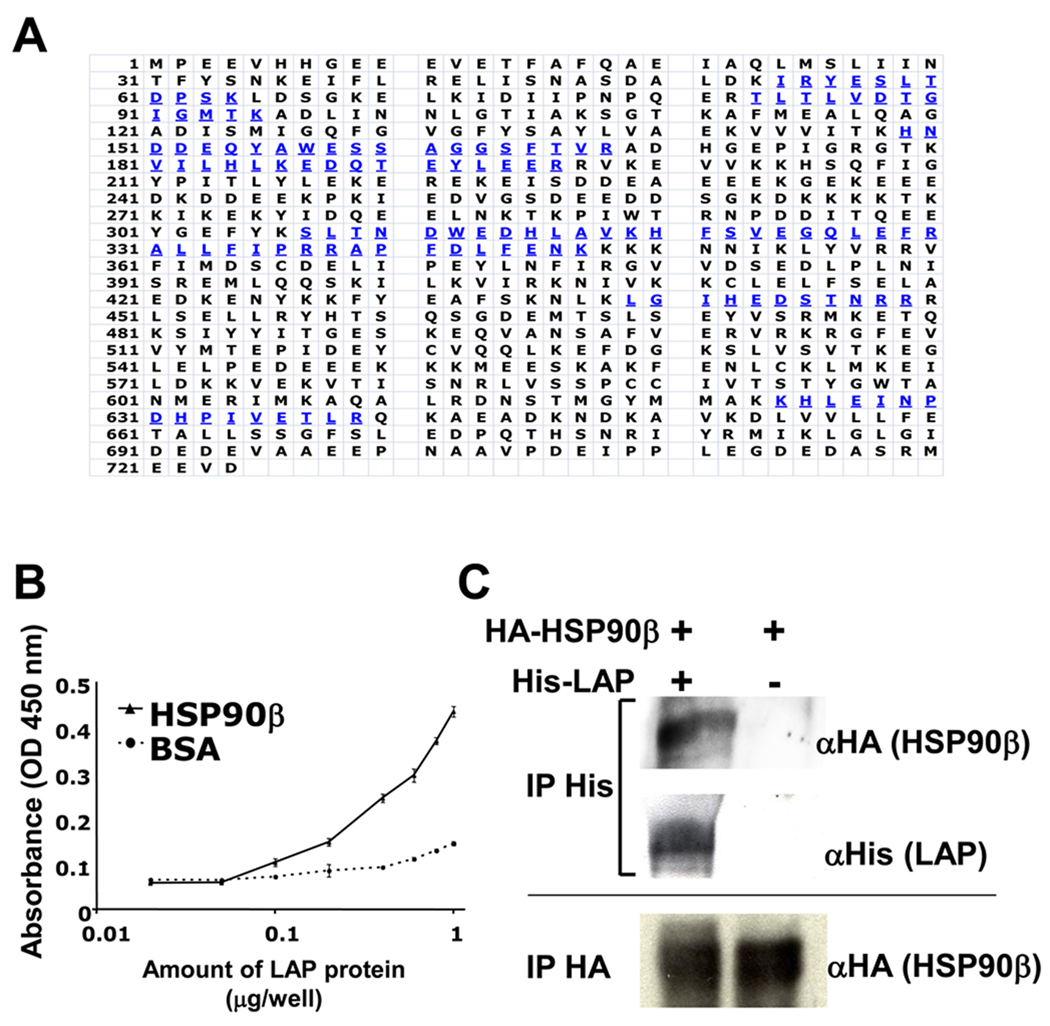
Figure 1. Isolation of HSP90β as LAP binding protein from MG63 cells.
A, The single intense band around 90 kDa is collected and trypsinized, and then sequenced by mass spectrometry. Blue amino acids were the sequences identified by mass spectrometry. B, 96-well plates were coated with HSP90β or BSA (100 µg/well). Coated plates were then incubated with serial concentrations of LAP proteins. The amount of binding LAP protein was determined by the anti-LAP antibody. Each value represents the mean of triplicate determinations; bars, SD. C, 293/EBNA and 293/EBNA-His-LAP cells were transiently transfected with HSP90β-HA. Supernatant was immunoprecipitated (IP) with the anti-His antibody (1st and 2nd rows) or anti-HA antibody (3rd lane), and then immunoblotted with anti-HA antibody (1st and 3rd rows) or anti-His antibody (2nd row).