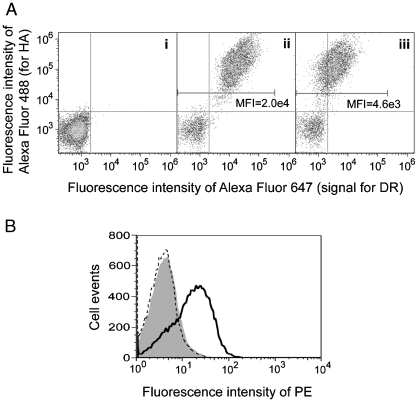
Fig. 2.
Expression of functional HLA-DR1 heterodimer in yeast by surface display. (A). Aga2p and HLA-DR1 levels of yeast expressing (i) an irrelevant soluble protein, (ii) Aga2p-fused HLA-DR1 with FLU-covalently linked to the β chain N-terminus, or (iii) empty Aga2p-fused HLA-DR1. Cells were double-labeled with reagents specific for the HA epitope tag (Aga2p level) and HLA-DRα. Mean fluorescence intensity (MFI) of DR signal for HA positive cell population was indicated. (B) Peptide-binding capability of yeast-displayed HLA-DR1. Yeast displaying empty HLA-DR1 (solid curve) or an irrelevant protein (shaded) were incubated with 100 μM biotinylated FLU at pH 5.0; HLA-DR1-displaying yeast were also incubated with 100 μM biotinylated control peptide (dashed curve). All samples were stained with streptavidin-PE and analyzed by flow cytometry.