Abstract
Adaptive immunity in jawless vertebrates is mediated by leucine-rich repeat proteins called “variable lymphocyte receptors” (VLRs). Two types of VLR (A and B) are expressed by mutually exclusive lymphocyte populations in lamprey. VLRB lymphocytes resemble the B cells of jawed vertebrates; VLRA lymphocytes are similar to T cells. We determined the structure of a high-affinity VLRA isolated from lamprey immunized with hen egg white lysozyme (HEL) in unbound and antigen-bound forms. The VLRA–HEL complex demonstrates that certain VLRAs, like γδ T-cell receptors (TCRs) but unlike αβ TCRs, can recognize antigens directly, without a requirement for processing or antigen-presenting molecules. Thus, these VLRAs feature the nanomolar affinities of antibodies, the direct recognition of unprocessed antigens of both antibodies and γδ TCRs, and the exclusive expression on the lymphocyte surface that is unique to αβ and γδ TCRs.
Keywords: crystal structure, T-cell receptor, variable lymphocyte receptor, evolution, antigen binding
The ability to mount specific immune responses to a virtually unlimited variety of antigens is unique to vertebrates. In jawed vertebrates, antigen recognition is mediated by antibodies and T-cell receptors (TCRs), which are expressed by B and T lymphocytes, respectively. In jawless vertebrates, adaptive immunity is mediated by antigen receptors that are fundamentally different from those of jawed vertebrates. Antibodies and TCRs are composed of Ig domains, but the variable lymphocyte receptors (VLRs) of jawless fish (lamprey and hagfish) consist of leucine-rich repeat (LRR) modules (1 –5). Like antibodies and TCRs, VLRs are generated in lymphocytes by DNA recombination. However, antibodies and TCRs are assembled from V, D, and J gene segments, and VLRs are assembled from multiple LRR-encoding cassettes selected from several hundred that flank each germline VLR gene (2, 6, 7). This process can generate a vast repertoire of receptors, estimated to comprise more than 1014 unique VLRs, which is sufficiently diverse to recognize most, if not all, pathogens (7 –9).
There are two types of VLRs, denoted “A” and “B,” which are expressed by mutually exclusive lymphocyte populations through monoallelic assembly of VLRA or VLRB genes (1, 2, 7). VLRB lymphocytes resemble the B cells of jawed vertebrates, whereas VLRA lymphocytes are surprisingly similar to T cells (10). Thus, VLRB lymphocytes respond to antigenic stimulation by proliferating and differentiating into plasmacytes that secrete VLRBs specific for native antigens. Like IgM antibodies, VLRBs overcome their weak monomeric affinities (micromolar K Ds) by forming high-avidity multimers (5, 11). By contrast, VLRA lymphocytes do not secrete their receptors following antigen activation. Like TCRs, VLRAs are expressed solely as cell-surface proteins (10). Furthermore, VLRA lymphocytes respond preferentially to classical T-cell mitogens, and a number of genes selectively expressed by VLRA lymphocytes are orthologs of genes typically expressed by T cells. These orthologs include genes encoding several transcription factors (GATA2/3, c-REL, BCL11b), the T-cell fate-determining molecule Notch1, the tyrosine phosphatase receptor protein CD45, the CCR9 chemokine receptor, two proinflammatory cytokines (IL-17 and MIF), and the IL-8 receptor CXCR2. This discovery of T-like and B-like lymphocytes in lamprey suggests that adaptive immunity is compartmentalized into cellular and humoral responses in both jawed and jawless vertebrates (6, 10).
Recently, crystal structures of VLRBs bound to H-antigen trisaccharide and hen egg white lysozyme (HEL) were reported (3, 5), showing how VLRB lymphocytes bind carbohydrate and protein antigens. By contrast, the mode of antigen recognition by VLRA lymphocytes is unknown. One possibility is that VLRA lymphocytes recognize processed antigens in a manner analogous to αβ T cells (12). However, jawless vertebrates lack genes encoding the MHC or MHC-like molecules used to present peptides or glycolipids to T cells in jawed vertebrates (6), although other presenting scaffolds cannot be excluded. Another possibility is that VLRA lymphocytes are functionally more akin to γδ T cells, which recognize antigens directly without any requirement for processing (12 –14). It also is conceivable that some VLRAs recognize only processed antigens, whereas others bind antigens directly. To investigate the basis for antigen recognition by the T-like lymphocytes of lamprey, we determined the structure of a VLRA in unbound and antigen-bound forms.
Results and Discussion
Overview of the VLRA–HEL Complex.
To access the VLRA repertoire, we used yeast surface display, as described previously (15). A VLRA library was constructed from lymphocyte cDNA of an HEL-immunized adult lamprey and screened for HEL binders. Thirteen closely related clones were isolated that appear to derive from a single lymphocyte precursor as a consequence of somatic diversification. Remarkably, these VLRAs exhibited affinities in the nano- to picomolar range, much higher than the micromolar affinities of HEL-specific VLRBs isolated from the same immunized lamprey (15).
We determined the structure of one of these VLRAs (VLRA.R2.1) in complex with HEL to 1.95-Å resolution (Table S1). As measured by surface plasmon resonance, soluble monomeric VLRA.R2.1 binds HEL with a dissociation constant (K D) of 180 pM (15), approaching the 100-pM affinity ceiling of Ig-based antibodies produced during mammalian immune responses (16). By contrast, this affinity far exceeds those of αβ TCRs for peptide–MHC ligands (1–100 μM) (17). VLRA.R2.1 adopts a horseshoe-shaped solenoid fold similar to that of VLRBs (Fig. 1 A and B) (3 –5). The structure comprises an N-terminal LRR capping module (LRRNT), six LRR modules (LRR1, LRRV1 through LRRV4, and LRRVe), a truncated LRR designated the connecting peptide (CP), and a C-terminal LRR capping module (LRRCT). The concave surface of VLRA.R2.1, through which the VLR binds HEL, is composed of nine parallel β-strands (two from LRRNT, six from LRRs, and one from CP) (Fig. 1A). Additional contacts are mediated by a protruding loop of LRRCT (residues 211–220), which corresponds to the highly variable insert of VLRs (9). This insert is absent from the LRRCTs of all other LRR-containing proteins except platelet receptor glycoprotein Ibα (GpIbα) (18).
Fig. 1.
Structure of the VLRA.R2.1–HEL complex and comparison with a VLRB–HEL complex. (A) Ribbon diagram of the VLRA.R2.1–HEL complex showing the concave antigen-binding surface of the VLR solenoid. LRRNT, green; LRR1, LRRV1–4, and LRRVe, magenta; CP, blue; LRRCT, orange; HEL, cyan. The LRRCT insert flanks the antigen. (B) Structure of the VLRB.2D–HEL complex (PDB accession code 3G3A) (5). The orientation of VLRB.2D is similar to that of VLRA.R2.1 in A. The LRRCT insert penetrates into the active site cleft of HEL.
The VLRA.R2.1–HEL complex buries a total surface area of 1,810 Å2, comparable to the surface buried in the VLRB.2D–HEL complex (1,685 Å2) (5), even though VLRB.2D is shorter by three LRRV modules, or in antibody–antigen (19) and TCR–MHC complexes (1,400–2,300 Å2) (13, 20). However, the shape complementarity of the VLRA.R2.1–HEL interface is considerably greater than that of the VLRB.2D–HEL interface, based on shape correlation statistics (S c) of 0.76 and 0.67, respectively (S c = 1.0 for interfaces with geometrically perfect fits) (21); this complementarity may explain, in part, the 2,400-fold higher affinity of VLRA.R2.1 compared with VLRB.2D (15). Indeed, an S c value of 0.76 is at the upper end of the range for protein–protein, including antibody–antigen, complexes.
VLRA–HEL Interface.
In the complex, 26 VLRA.R.2.1 residues interact with 18 HEL residues (Table 1). The ligand-binding site spans the entire concave face of VLRA.R2.1, from LRRNT to LRRCT (Fig. 1A). Of the total buried surface in the VLRA.R2.1–HEL complex, the LRRNT, LRR (LRR1, LRRV1–4 and LRRVe), CP, and LRRCT modules contribute 5%, 50%, 12%, and 33%, respectively (Fig. 2A), compared with 0%, 27%, 15%, and 58%, respectively, in the VLRB.2D–HEL complex (Fig. 2B). Hence, the HEL-contacting surface of VLRA.R2.1 derives predominantly from the β-strands of the VLR (67% of total buried surface), whereas the LRRCT module contributes proportionately more to the HEL-contacting surface of VLRB.2D (58% of total buried surface). This difference results mainly from contacts between HEL and a region between the LRRCT insert and the strand dyad of LRRCT closest to the C terminal, contacts that are present only in the VLRB.2D–HEL complex.
Table 1.
Interactions between VLRA.R2.1 and HEL
| VRLA.R2.1 | HEL | |
| Hydrogen bonds | Van der Waals contacts | |
| Q21 | Q21(Oε1)–R61(Nη1, Nη2) | R61 |
| Q42 | Q42(Nε2)–D48(Oδ2) | D48, R61 |
| W62 | T47 | |
| N64 | T47 | |
| D66 | D66(Oδ2)–T47(Oγ1) | T47 |
| Y67 | V109 | |
| G88 | T47 | |
| N91 | V109 | |
| Y112 | Y112(OH)–N46(O) | R45, N46, T47 |
| G115 | V109 | |
| R136 | R136(Nη1, Nη2)–R45(O) | R45 |
| T139 | V109, A110, N113 | |
| N140 | N140(O)–N113(Nδ2) | N113 |
| T163 | T163(Oγ1) –F34(O) | F34, N113, R114 |
| N164 | N164(O)–R114(Nη2) | R114 |
| Q165 | Q165(Nε2)–R114(Nη2) | R114 |
| F186 | K33, F34, N37 | |
| G187 | G187(O)–R114(Nη1, Nη2) | F34, R114 |
| Q189 | R114 | |
| D213 | D213(Oδ1)–N37(Oδ1) | S36, N37, N39, A42 |
| D213(Oδ2)–N39(Nδ2) | ||
| G214 | T43, N44 | |
| T215 | T215(N)–T43(O) | T43, N44 |
| T215(N)–N44(Oδ1) | ||
| T215(Oγ1) –N44(Oδ1) | ||
| G216 | G216(N)–T43(O) | T43 |
| L219 | Q41, A42 | |
| S222 | N39 | |
| G225 | G225(O)–N37(Oδ1) | N37 |
Hydrogen bonds were calculated using a cutoff distance of 3.4 Å. The cutoff distance for van der Waals contacts was 4.0 Å.
Fig. 2.
Surface analysis of VLRA–HEL, VLRB–HEL, and antibody–HEL interfaces. Residues involved in contacts are depicted as surface representations. (A) Binding surface of VLRA.R2.1 with contacting residues from LRRNT in green, from LRR1, LRRV1–4, and LRRVe in magenta, from CP in blue, and from LRRCT in orange. (B) Binding surface of VLRB.2D in the complex with HEL. (C) Comparison of VLRA and VLRB epitopes on HEL. Residues that interact exclusively with VLRA.R2.1 in the VLRA.R2.1–HEL complex are pink; residues that interact exclusively with VLRB.2D in the VLRB.2D–HEL complex are yellow; residues forming contacts in both complexes are red. (D) Antibody epitopes on HEL. The orientation of HEL is the same as in C. The epitopes for mouse antibodies D1.3, HyHEL-5, and HyHEL-10 (35) are green, beige, and brown, respectively. The epitope for camel antibody cAb-Ly3 (23) is cyan.
Although LRRNT does not contact ligand in the two VLRB–antigen complexes (3, 5), LRRNT of VLRA.R2.1 engages HEL through Gln21, which forms two side-chain–side-chain hydrogen bonds with HEL Arg61 (Fig. 3A and Table 1). However, these interactions contribute little to productive binding, because mutation of Gln21 to alanine did not appreciably affect affinity, as measured by yeast surface titration (Fig. 4). By contrast, LRRCT of VLRA.R2.1 interacts extensively with HEL via its 10-residue insert (Val211–His220), which makes six hydrogen bonds and 37 van der Waals contacts with HEL residues 36–44 (Fig. 3B and Table 1). Moreover, the LRRCT insert is critical for complex formation, as shown below.
Fig. 3.
The VLRA.R2.1–HEL binding interface. (A) Close-up view of contacting residues in the VLRA.R2.1–HEL interface. Colors (including residue labels) are as in Fig. 1. For clarity, only interactions involving selected residues from LRRNT, LRR1, and LRRV1–4 are shown. The side chains of contacting residues are drawn in ball-and-stick representation, with nitrogen atoms in blue and oxygen atoms in red. Hydrogen bonds are indicated by dotted lines. A bound water molecule bridging VLRA.R2.1 Arg136 and HEL Arg45 is represented as a red sphere. (B) Interactions between the LRRCT module of VLRA.R2.1 and HEL. Only side-chain–side-chain hydrogen bonds are depicted.
Fig. 4.
Mutational analysis of VLRA binding to HEL. Several HEL-contacting residues of VLRA.R2.1 were subjected to mutational analysis. Replacement of Arg136 of LRRV4 by tryptophan (VLRA.R2.1 R136W, blue) reduced the affinity of VLRA.R2.1 for HEL (green) 72-fold (K D from 650 pM to 47 nM). Mutation of Trp136 to arginine in VLRA.R3.1 (VLRA.R3.1 W136R, red), the putative germline progenitor of VLRA.R2.1, increased its affinity 108-fold, from K D = 34 nM (VLRA.R3.1, black) to K D =316 pM. Mutation of LRRNT residue Gln21 to alanine (VLRA.R2.1 Q21A, purple) decreased the affinity of VLRA.R2.1 for HEL 1.4-fold (K D = 922 pM). Alanine substitution of LRRCT residues Asp213 and Leu219 abolished binding, even up to 100 nM HEL. By contrast, mutation of Gln217 (VLRA.R2.1 Q217A, yellow) had little effect (K D = 544 pM).
The antigen-binding concave face of VLRA.R2.1, like that of VLRBs (5), is composed of three parallel ridges, R1, R2, and R3, associated with the beginning, middle, and end, respectively, of the β-strand of the LRR modules (Fig. S1 A and B). Each β-strand contains a six-residue motif, X1L(I)X3LX5X6, in which leucine or isoleucine at positions 2 and 4 compose the hydrophobic core of the VLR and the remaining four residues form the three ridges: R1 (position 1), R2 (position 3), and R3 (positions 5 and 6). The residues of R1, R2, and R3 involved in ligand binding in the three known VLR–antigen structures are highlighted in Fig. S1. The distribution of contacting residues on the ridges is highly variable, depending on the nature and positioning of the ligand. Involvement can range from full engagement, as for R3 of VLRA.R2.1 bound to HEL (Fig. S1A), to no contacts whatsoever, as for R1 of VLR RBC36 bound to H-antigen trisaccharide (Fig. S1C). Such differential engagement is reminiscent of antibodies or TCRs, which bind ligands using all six complementarity-determining region (CDR) loops or only subsets of these loops (19, 20).
Basis for Binding Affinity.
VLRA.R2.1 differs from its putative germline sequence at eight amino acid positions: five in LRRNT, one in LRRV4, one in CP, and one in LRRCT (15). However, only the substitution in LRRV4 (Trp/Arg136) is located in the interface with HEL, where the side chain of Arg136 forms two direct and one water-mediated hydrogen bonds to the main-chain oxygen of HEL Arg45 (Fig. 3A and Table 1). Replacement of Arg136 by tryptophan (the germline residue) would eliminate these interactions and possibly alter the conformation of the LRRCT insert, which is proximal to Arg136. Indeed, this mutation reduced the affinity of VLRA.R2.1 for HEL 72-fold (Fig. 4), effectively matching that of VLRA.R3.1 which possesses the germline sequence. Conversely, mutation of Trp136 to arginine in VLRA.R3.1 increased its affinity 108-fold, demonstrating that Arg136 largely accounts for the substantial difference in affinity between VLRA.R2.1 and its putative germline progenitor.
Conformational Changes upon Complex Formation.
To identify possible conformational changes in VLRA.R2.1 associated with antigen binding, we determined the structure of a closely related HEL-specific VLRA, VLRA.R5.1, in unbound form to 1.70-Å resolution (Table S1). This VLRA differs from VLRA.R2.1 by only a single residue, isoleucine instead of threonine at position 197, which does not contact antigen in the VLRA.R2.1–HEL complex but rather points toward the hydrophobic core of LRRCT. Moreover, VLRA.R5.1 and VLRA.R2.1 bind HEL with nearly identical K Ds (120 pM and 180 pM, respectively) (15). Superposition of free VLRA.R5.1 onto VLRA.R2.1 in complex with HEL gave an rms deviation of 0.9 Å for all atoms, indicating close similarity, even at the level of individual side chains (Fig. 5). However, 7 of 10 residues of the LRRCT insert were completely disordered in unbound VLRA.R5.1, suggesting conformational flexibility. By contrast, continuous and unambiguous electron density was observed for the entire LRRCT insert in the VLRA.R2.1–HEL complex. Given the apparent mobility of the LRRCT insert and its flanking location in the interface with HEL, we asked whether the insert contributed significantly to binding. Strikingly, alanine substitution of Asp213 and Leu219, which make hydrogen bonding and/or van der Waals contacts with HEL (Table 1), completely abrogated binding. By contrast, mutation of Gln217, a noncontacting residue at the tip of LRRCT insert, had no appreciable effect (Fig. 4).
Fig. 5.
Comparison of unbound and HEL-bound VLRAs. Superposition of unbound VLRA.R5.1 (yellow) onto VLRA.R2.1 (magenta) in complex with HEL (cyan). VLRA.R5.1 differs from VLRA.R2.1 by only a single residue (Ile/Thr197), which is distant from HEL. In unbound VLRA.R5.1, only 2 of 10 residues of the LRRCT insert are visible in electron density: Asp213 and Ser222. The side chains of residues showing the largest conformational differences between VLRA.R5.1 and VLRA.R2.1, along with the HEL residues they contact, are drawn in ball-and-stick format.
These results suggest a mechanism for VLRA binding based on conformational selection from a dynamic equilibrium of preexisting isomers, as proposed for antibodies and TCRs (22, 23). According to this mechanism, the intrinsically mobile LRRCT insert samples multiple conformations, only one of which is competent to bind a specific ligand. Such conformational diversity could enable a single receptor to engage multiple ligands, thereby expanding the effective size of the VLRA repertoire. Similar considerations may apply to VLRBs.
Comparison of the HEL Epitope Recognized by VLRA.R2.1 with Other HEL Epitopes.
VLRA.R2.1 recognizes a flat epitope on HEL with the 10-residue LRRCT insert packing against one side of the antigen (Fig. 1A) in a manner resembling the interaction of GpIbα with its protein ligand, von Willebrand factor (18). By contrast, VLRB.2D targets a concave epitope on HEL, with its six-residue LRRCT insert projecting deep into the carbohydrate-binding cleft of the enzyme (Fig. 1B) (5). These two epitopes are essentially distinct, despite some overlap (only 5 of 18 HEL residues contacted by VLRA.R2.1 are also contacted by VLRB.2D) (Fig. 2C). The VLRA.R2.1 epitope also is distinct from those recognized by all HEL-specific antibodies characterized to date (Fig. 2D) (19), implying significant differences in the antigenic structure of proteins in jawed and jawless vertebrates.
In jawed vertebrates, the two-domain VLVH antibodies of humans and mice, whose binding sites are relatively flat, exhibit a strong proclivity for planar epitopes (19, 24), whereas the single-domain VH antibodies of camels and sharks, with their convex binding sites, preferentially target clefts not accessible to VLVH antibodies (24 –26). By recognizing both flat (VLRA.R2.1) and concave (VLRB.2D) epitopes, the VLRs of jawless vertebrates seem to have evolved to bind as topologically diverse an array of protein epitopes as VLVH and VH antibodies combined but do so using only one basic structure, a monomeric LRR solenoid.
Correlation Between Antigen Binding and Sequence Variability of VLRAs.
Structure-based sequence comparisons of VLRAs and VLRBs revealed clear differences between these two types of VLR, suggesting possible specialization in terms of antigen recognition. In both lamprey and hagfish, VLRAs display much less variation in the length of the LRRCT insert than do VLRBs (5); this difference recalls the constrained length distribution of the CDR3s of αβ TCRs as compared with antibody CDR3s (27). VLRAs also appear to have a potentially larger antigen-binding surface, on average, than VLRBs: Whereas an average VLRA from lamprey or hagfish contains ∼3 LRRV modules, VLRBs from lamprey include an average of 1.3 LRRV, and those from hagfish contain ∼2 LRRVs.
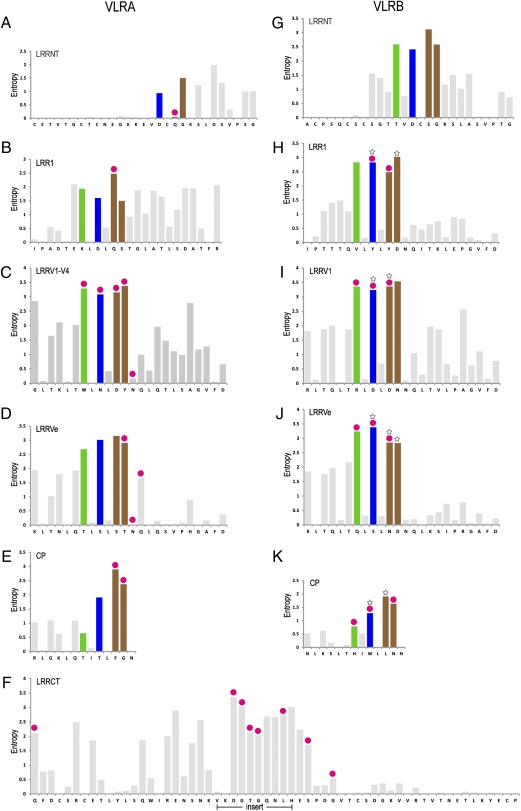
To examine the sequence variability of VLRAs, we combined structural information with sequence analysis of a comprehensive database of LRR modules from 208 lamprey VLRA sequences. We prepared multiple sequence alignments of all LRRs corresponding to the HEL-contacting LRR modules of VLRA.R2.1 and calculated Shannon entropy (28) for each aligned position as an objective measure of sequence diversity. As in VLRBs (5), the most variable positions in the LRRs (relative entropy > 2.0) are distributed mainly along the three parallel ridges on the concave face of VLRAs (Fig. 6 A–E) or are located in the LRRCT insert (Fig. 6F). However, in terms of specific features, VLRAs are clearly distinguished from VLRBs. Thus, the first two LRRs of VLRAs (LRRNT and LRR1, but especially LRRNT) have a paucity of high-entropy positions—a total of three in VLRAs (Fig. 6 A and B) compared with eight in VLRBs (Fig. 6 G and H), only one of which (in LRR1) is located on the concave antigen-binding surface. In addition, VLRAs display more high-entropy positions on the convex surface opposite the binding surface than do VLRBs. As in VLRBs (Fig. 6 G–K ), we observed a strong correlation between the highest-entropy and ligand-contacting positions, such that 14 of 31 positions with entropy > 2.0 also contact antigen in the VLRA.R2.1–HEL structure [most notably the top four high-entropy positions in the LRRVs (Fig. 6C) and five of the eight highest-entropy positions in LRRCT inserts (Fig. 6F)]. However, compared with VLRBs, a larger number of low-entropy positions were found to contact the ligand, including several invariant or nearly invariant residues in LRRNT, the LRRVs, and LRRVe (Fig. 6 A, C, and D). The most intriguing of these differences is the far lower sequence diversity of LRRNT in VLRAs than in VLRBs (Fig. 6 A and G), because this reduced diversity could imply the existence of conserved interactions between LRRNT and accessory molecules that may be uniquely required for activating T-like VLRA lymphocytes.
Fig. 6.
Sequence variability and antigen-contacting positions of VLRAs and VLRBs. (A–F) Sequence diversity plots for VLRAs. Shannon entropy per aligned position is shown for LRRNT, LRR1, LRRVs, LRRVe, CP, and LRRCT. The entropy was calculated from 208 lamprey VLRA sequences. Entropy bars corresponding to ridges R1, R2, and R3 are green, blue, and brown, respectively; all other bars are gray. HEL-contacting positions of VLRA.R2.1 are marked by red circles above the entropy bars. The VLRA.R2.1 sequence is shown below the plots as reference. (G–K) Sequence diversity plots for VLRBs. Shannon entropy per aligned position is indicated for LRRNT, LRR1, LRRVs, LRRVe, and CP. The entropy was calculated from 588 lamprey VLRB sequences. Red circles above the entropy bars mark the HEL-contacting positions on VLRB.2D; white stars indicate the H-trisaccharide–contacting positions of VLRB.RBC36. The sequence below is that of VLRB.2D.
Conclusions
The VRLA.R2.1–HEL complex demonstrates that at least certain VLRAs are capable of binding antigens directly without any apparent requirement for processing or assistance by specialized antigen-presenting molecules. In this respect, such VLRAs resemble antibodies and γδ TCRs, which, unlike αβ TCRs, recognize antigens directly (12, 14). For example, the mouse γδ TCR G8 binds directly to its ligand, the nonclassical MHC molecule T22, in the absence of antigen processing (13). In the complex, G8 contacts T22 mainly through its long δ-chain CDR3 loop, a recognition mode that is distinct from that of αβ TCRs but reminiscent of the binding of VH antibodies, which also engage antigen primarily through CDR3 (24 –26). Although VLRA.R2.1 and VLRB.2D target different epitopes of HEL, they use similar overall strategies for direct antigen recognition.
Remarkably, VRLA.R2.1 and other VLRAs isolated from HEL-immunized lamprey exhibit affinities in the nano- to picomolar range (15), comparable to those of affinity-matured IgG antibodies and much higher than the micromolar affinities of αβ or γδ TCRs (13, 17). Therefore, these VLRAs combine certain features of antibodies (nanomolar K Ds) or features of both antibodies and γδ TCRs (direct recognition of unprocessed antigen) with other features that are unique to αβ and γδ TCRs (exclusive expression on the lymphocyte surface). It also is noteworthy that HEL-specific VLRBs, derived from the same immunized lamprey as VLRA.R2.1, bound HEL with micromolar K Ds (15). However, VLRBs, like IgM antibodies, overcome their low monomeric affinities for antigen by forming high-avidity multimers in plasma (5, 11). Such a compensatory mechanism may not exist for VLRAs, whose oligomerization state on the lymphocyte surface is unknown; the absence of a compensatory mechanism might explain their high monomeric affinities, at least for soluble antigens.
Phylogenetic analysis of TCR sequences has provided evidence that γδ TCRs are evolutionarily more ancient than αβ TCRs and that the primordial antigen receptors of jawed vertebrates were γδ-like (29). This analysis implies that γδ T cells exhibit the primitive condition of direct antigen binding, whereas indirect, MHC-restricted antigen recognition is a derived characteristic of αβ T cells. We propose that the direct-binding VLRAs characterized here represent functional analogs of γδ TCRs. Whether jawless vertebrates evolved other VLRA subsets to recognize antigens indirectly, possibly through presentation by a convergent analog of the MHC molecule, remains to be established.
Materials and Methods
Protein Preparation.
VLRA.R2.1 and VLRA.R5.1 were isolated from a VLRA library (5 × 107 independent clones) generated from lymphocyte cDNA of an HEL-immunized adult lamprey and displayed on the surface of yeast (15). The library was sorted by flow cytometry with fluorescent-labeled HEL, and 13 unique clones were isolated, including VLRA.R2.1 and VLRA.R5.1. The diversity regions of VLRA.R2.1 and VLRA.R5.1, from LRRNT to LRRCT, were expressed by in vitro folding from bacterial inclusion bodies (SI Materials and Methods).
Crystallization and Structure Determination.
Crystals of the VLRA.R2.1–HEL complex (20 mg/mL) were obtained at room temperature in 15% (wt/vol) polyethylene glycol 2000 and 0.1 M Bis-Tris (pH 7.0). Crystals of unbound VLRA.R5.1 grew in 20% (wt/vol) polyethylene glycol 6000, 1.0 M LiCl, and 0.1 M citric acid (pH 4.0). For data collection, crystals of VLRA.R2.1–HEL and VLRA.R5.1 were cryoprotected with 20% (wt/vol) glycerol before flash cooling in liquid nitrogen. X-ray diffraction data were collected at beamline ×29 of the Brookhaven National Synchrotron Light Source with an ADSC Quantum-315 CCD detector. All data were indexed, integrated, and scaled with the HKL2000 program (30). The structures of VLRA.R2.1–HEL and VLRA.R5.1 were determined by molecular replacement (SI Materials and Methods). Data collection and refinement statistics are presented in Table S1. Atomic coordinates and structure factors for the VLRA.R2.1–HEL complex and VLRA.R5.1 have been deposited in the Protein Data Bank (PDB) under accession codes 3M18 and 3M19, respectively.
Equilibrium Dissociation Constants.
Yeast surface antigen titrations of VLRAs were performed as reported (15). Triplicate aliquots of 5 × 105 yeast cells were labeled with HEL concentrations spanning at least 2 orders of magnitude, both above and below the dissociation constant. Equilibrium dissociation constants were obtained by plotting total mean fluorescence (PE channel) against antigen concentration, using nonlinear least squares to fit the curve.
Sequence Analysis.
All VLR sequences used here were derived from previous studies (1, 2, 9). Protein sequence searches were performed using the BLASTPGP and PSI-BLAST programs (31), with a profile threshold of 0.01. Similarity-based clustering of protein sequences was performed with BLASTCLUST (ftp://ftp.ncbi.nih.gov/blast). Multiple sequence alignments were constructed with KALIGN (32). Structural alignments were performed using MUSTANG (33), and the structures were rendered using PYMOL (http://www.pymol.org/) and SWISS-PDBviewer (http://spdbv.vital-it.ch/). Ligand-binding residues were determined using a custom script that searches for atoms within spheres of specified radius, such as 3.0 Å or 5.0 Å. Entropy calculations were performed with a custom script using the alignments generated by KALIGN (34). The entropy was calculated using the Shannon entropy formula:  , where Pi is the fraction of a given amino acid i, and M the total number of different amino acids.
, where Pi is the fraction of a given amino acid i, and M the total number of different amino acids.
Supplementary Material
Acknowledgments
We thank H. Robinson (Brookhaven National Synchrotron Light Source) for X-ray data collection. Support for beamline X29 comes from the Offices of Biological and Environmental Research and of Basic Energy Sciences of the US Department of Energy and from the National Center for Research Resources of the National Institutes of Health. This study was supported by National Institutes of Health Grants AI065610 (to R.A.M.), AI083892 (to Z.P. and R.A.M.), and RR006603 (to M.F.F.) and by National Science Foundation Grant MCB-0614672 (to Z.P.). L.M.I. and L.A. were supported by the National Library of Medicine of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: Atomic coordinates and structure factors for the VLRA.R2.1-HEL complex and VLRA.R5.1 have been deposited in the Protein Data Bank under accession codes 3M18 and 3M19, respectively.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005475107/-/DCSupplemental
References
- 1.Pancer Z, et al. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–180. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- 2.Alder MN, et al. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science. 2005;310:1970–1973. doi: 10.1126/science.1119420. [DOI] [PubMed] [Google Scholar]
- 3.Han BW, Herrin BR, Cooper MD, Wilson IA. Antigen recognition by variable lymphocyte receptors. Science. 2008;321:1834–1837. doi: 10.1126/science.1162484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HM, et al. Structural diversity of the hagfish variable lymphocyte receptors. J Biol Chem. 2007;282:6726–6732. doi: 10.1074/jbc.M608471200. [DOI] [PubMed] [Google Scholar]
- 5.Velikovsky CA, et al. Structure of a lamprey variable lymphocyte receptor in complex with a protein antigen. Nat Struct Mol Biol. 2009;16:725–730. doi: 10.1038/nsmb.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: Genetic events and selective pressures. Nat Rev Genet. 2010;11:47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pancer Z, Cooper MD. The evolution of adaptive immunity. Annu Rev Immunol. 2006;24:497–518. doi: 10.1146/annurev.immunol.24.021605.090542. [DOI] [PubMed] [Google Scholar]
- 8.Alder MN, et al. Antibody responses of variable lymphocyte receptors in the lamprey. Nat Immunol. 2008;9:319–327. doi: 10.1038/ni1562. [DOI] [PubMed] [Google Scholar]
- 9.Rogozin IB, et al. Evolution and diversification of lamprey antigen receptors: Evidence for involvement of an AID-APOBEC family cytosine deaminase. Nat Immunol. 2007;8:647–656. doi: 10.1038/ni1463. [DOI] [PubMed] [Google Scholar]
- 10.Guo P, et al. Dual nature of the adaptive immune system in lampreys. Nature. 2009;459:796–801. doi: 10.1038/nature08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrin BR, et al. Structure and specificity of lamprey monoclonal antibodies. Proc Natl Acad Sci USA. 2008;105:2040–2045. doi: 10.1073/pnas.0711619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis MM, Chien YH. In: Fundamental Immunology. Paul WE, editor. Philadelphia: Lippincott Williams & Wilson; 2008. pp. 313–345. [Google Scholar]
- 13.Adams EJ, Chien YH, Garcia KC. Structure of a gammadelta T cell receptor in complex with the nonclassical MHC T22. Science. 2005;308:227–231. doi: 10.1126/science.1106885. [DOI] [PubMed] [Google Scholar]
- 14.Konigshofer Y, Chien YH. Gammadelta T cells—innate immune lymphocytes? Curr Opin Immunol. 2006;18:527–533. doi: 10.1016/j.coi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Tasumi S, et al. High-affinity lamprey VLRA and VLRB monoclonal antibodies. Proc Natl Acad Sci USA. 2009;106:12891–12896. doi: 10.1073/pnas.0904443106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batista FD, Neuberger MS. Affinity dependence of the B cell response to antigen: A threshold, a ceiling, and the importance of off-rate. Immunity. 1998;8:751–759. doi: 10.1016/s1074-7613(00)80580-4. [DOI] [PubMed] [Google Scholar]
- 17.van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- 18.Huizinga EG, et al. Structures of glycoprotein Ibalpha and its complex with von Willebrand factor A1 domain. Science. 2002;297:1176–1179. doi: 10.1126/science.107355. [DOI] [PubMed] [Google Scholar]
- 19.Sundberg EJ, Mariuzza RA. Molecular recognition in antibody-antigen complexes. Adv Protein Chem. 2002;61:119–160. doi: 10.1016/s0065-3233(02)61004-6. [DOI] [PubMed] [Google Scholar]
- 20.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence MC, Colman PM. Shape complementarity at protein/protein interfaces. J Mol Biol. 1993;234:946–950. doi: 10.1006/jmbi.1993.1648. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong KM, Piepenbrink KH, Baker BM. Conformational changes and flexibility in T-cell receptor recognition of peptide-MHC complexes. Biochem J. 2008;415:183–196. doi: 10.1042/BJ20080850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James LC, Roversi P, Tawfik DS. Antibody multispecificity mediated by conformational diversity. Science. 2003;299:1362–1367. doi: 10.1126/science.1079731. [DOI] [PubMed] [Google Scholar]
- 24.De Genst E, et al. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc Natl Acad Sci USA. 2006;103:4586–4591. doi: 10.1073/pnas.0505379103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanfield RL, Dooley H, Flajnik MF, Wilson IA. Crystal structure of a shark single-domain antibody V region in complex with lysozyme. Science. 2004;305:1770–1773. doi: 10.1126/science.1101148. [DOI] [PubMed] [Google Scholar]
- 26.Stanfield RL, Dooley H, Verdino P, Flajnik MF, Wilson IA. Maturation of shark single-domain (IgNAR) antibodies: Evidence for induced-fit binding. J Mol Biol. 2007;367:358–372. doi: 10.1016/j.jmb.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 27.Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capra JA, Singh M. Predicting functionally important residues from sequence conservation. Bioinformatics. 2007;23:1875–1882. doi: 10.1093/bioinformatics/btm270. [DOI] [PubMed] [Google Scholar]
- 29.Richards MH, Nelson JL. The evolution of vertebrate antigen receptors: A phylogenetic approach. Mol Biol Evol. 2000;17:146–155. doi: 10.1093/oxfordjournals.molbev.a026227. [DOI] [PubMed] [Google Scholar]
- 30.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 31.Altschul SF, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lassmann T, Sonnhammer EL. Kalign—an accurate and fast multiple sequence alignment algorithm. BMC Bioinformatics. 2005;6:298. doi: 10.1186/1471-2105-6-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konagurthu AS, Whisstock JC, Stuckey PJ, Lesk AM. MUSTANG: A multiple structural alignment algorithm. Proteins. 2006;64:559–574. doi: 10.1002/prot.20921. [DOI] [PubMed] [Google Scholar]
- 34.Lassmann T, Frings O, Sonnhammer EL. Kalign2: High-performance multiple alignment of protein and nucleotide sequences allowing external features. Nucleic Acids Res. 2009;37:858–865. doi: 10.1093/nar/gkn1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies DR, Sheriff S, Padlan EA. Antibody-antigen complexes. J Biol Chem. 1988;263:10541–10544. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.