Abstract
Pancreatic cancer is one of the most fatal malignancies lacking effective therapies. Notch signaling is a key regulator of cell fate specification and pancreatic cancer development; however, the role of individual Notch receptors and downstream signaling is largely unknown. Here, we show that Notch2 is predominantly expressed in ductal cells and pancreatic intraepithelial neoplasia (PanIN) lesions. Using genetically engineered mice, we demonstrate the effect of conditional Notch receptor ablation in KrasG12D-driven pancreatic carcinogenesis. Deficiency of Notch2 but not Notch1 stops PanIN progression, prolongs survival, and leads to a phenotypical switch toward anaplastic pancreatic cancer with epithelial–mesenchymal transition. By expression profiling, we identified increased Myc signaling regulated by Notch2 during tumor development, placing Notch2 as a central regulator of PanIN progression and malignant transformation. Our study supports the concept of distinctive roles of individual Notch receptors in cancer development.
Keywords: genetically engineered mice, K-Ras, Myc, Notch, pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) remains a devastating disease despite tremendous therapeutical efforts. PDAC derives from several preneoplastic lesions, including pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm, and mucinous cystic neoplasm (MCN), of which PanINs are the most common precursors (1). PanINs typically progress through defined histological and molecular stages, with the most advanced PanIN3 lesion being defined as carcinoma in situ (2). Because of early metastatic spread, PanIN3 represents the latest curable precursor lesion. Thus, defining the regulators of PanIN initiation and progression is of utmost importance.
Recapitulation of human pancreatic carcinogenesis was greatly advanced by generating mice with pancreas-specific activation of endogenous oncogenic KrasG12D (3). The ongoing characterization of relevant signaling pathways in pancreatic carcinogenesis using genetically engineered mouse models has helped to depict the enormous plasticity in precursors to PDAC. Despite activation of cell fate regulating signaling pathways such as Hedgehog, Wnt, and Notch signaling (3–9), the precise role of these pathways remains largely unclear.
The Notch signaling pathway plays a pivotal role in cell fate and differentiation decisions, and its activation early in the carcinogenic process suggests a role in initiation of transformation. Although the cell of origin in PDAC has not been decisively identified, activation of Notch signaling during PanIN initiation probably presents a pivotal step for transformation. In several murine models of PDAC, expression of the Notch target gene Hes1 was increased in PanIN lesions (3, 5, 8, 9). In a recent study, chemical inhibition of Notch activation completely blocked tumor progression in vivo (10). Conversely, Murtaugh and co-workers (11) described a PanIN-promoting effect of Notch activation in KrasG12D-driven PanIN development. However, the specific role of individual Notch receptors and the downstream events have so far not been determined.
Here, we describe the effect of pancreas-specific ablation of Notch1 and Notch2 in KrasG12D-driven pancreatic carcinogenesis, taking advantage of the nonessential role of Notch1 and Notch2 during pancreatogenesis (12). We show that Notch1 and Notch2 are expressed in pancreatic acinar and ductal cells, respectively. Conditional ablation of Notch2 but not Notch1 leads to an abrogation of PanIN progression, development of MCN-like lesions, and increased survival. Identification of Notch2-regulated Myc signaling during carcinogenesis points to a central role of Notch2 in controlling PanIN progression and tumor differentiation.
Results
Notch1 and Notch2 Are Expressed in Different Compartments in Adult Pancreata and Are Activated in Kras Mice During PanIN Development.
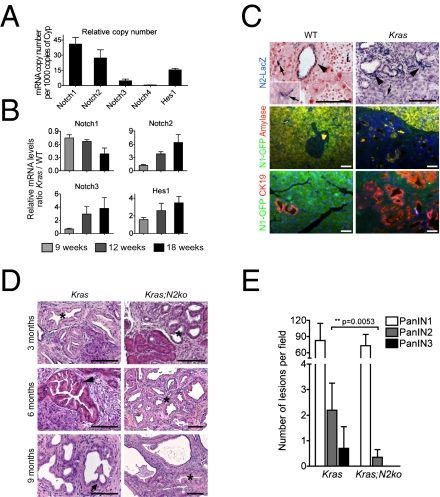
To determine the expression of members of the Notch signaling family during pancreatic carcinogenesis, Kras+/LSL-G12D mice were crossed to Ptf1a+/Cre(ex1) mice (referred to as Kras; Fig. S1C), as previously described (9). Notch1 and Notch2 were predominantly expressed in whole-tissue mRNA from WT and KrasG12D-induced pancreata compared with low expression of Notch3 and Notch4 (Fig. 1A). In Kras pancreata at 9 wk of age, when only a few PanIN1 lesions are notable, increased expression of Notch2 and the Notch target gene Hes1 but not Notch1 was observed, similar to previous reports (5). During progression, we noted a significant increase in Notch2 and Hes1 expression, whereas Notch1 was further reduced. Notch3 was also increased, albeit at lower total expression levels (Fig. 1B). This expression pattern correlated well with an increase in CK19 and a decrease in amylase expression, suggesting that Notch2 is expressed in CK19+ PanINs, whereas Notch1 may be predominantly expressed in acinar cells. To test this hypothesis, we used transgenic Notch1-GFP and Notch2lacZ knockin reporter mice (13, 14) to localize Notch1 and Notch2 expression in WT and Kras mice. In WT pancreata, we found X-Gal as a surrogate for Notch2 expression in ductal but not acinar or islet cells (Fig. 1C). Moreover, X-Gal+ cells were notable in the typical centroacinar position thought to be a presumed progenitor cell compartment (15) (Fig. 1C). In Kras:Notch2lacZ mice, X-Gal expression was detectable in PanIN lesions and the surrounding stroma (Fig. 1C). GFP expression as a surrogate for Notch1 was found in normal acinar cells, as previously described (16), but was hardly ever detectable in PanIN lesions (Fig. 1C). In summary, these expression data are consistent with Notch2 as the predominant Notch receptor in ductal, centroacinar, and PanIN cells as suggested previously (5).
Fig. 1.
Expression analysis of Notch receptors in WT and KrasG12D-induced pancreata. (A) Transcript levels of Notch receptors and Hes1 in relation to cyclophilin gene expression in WT pancreata (n = 3). (B) Quantification of Notch receptor and Hes1 gene expression at indicated time points in Kras pancreatic tissue. Values represent WT-to-Kras tissue ratios of relative expression levels (n = 4). (C) Expression of Notch1 and Notch2 in distinct compartments of 18-wk-old WT and Kras pancreas using Notch1 and Notch2 reporter mice. Arrows indicate centroacinar cells, and arrowheads point to X-Gal+ ducts and PanINs. i, islets. (D) H&E staining of 3-, 6-, and 9-mo-old Kras and Kras;N2ko pancreata. Asterisks indicate PanIN1, arrowhead points to PanIN2, and arrow indicates PanIN3 lesions. Note the absence of PanIN2/3 in Kras;N2ko mice. (Scale bars: 50 μm.) (E) Quantification of PanINs in 9-mo-old Kras (n = 4) and Kras;N2ko (n = 5) mice shows a significant reduction in PanIN2 and absence of PanIN3 lesions in Kras;N2ko mice.
PanIN Development and Progression in Notch-Ablated Pancreata.
To analyze the effect of Notch1 and Notch2 deficiency in pancreatic carcinogenesis, we crossed previously described floxed Notch1fl/fl and Notch2fl/fl mice (17) with Ptf1a+/Cre(ex1) mice (18) for generation of Ptf1a+/Cre(ex1);Notch1fl/fl and Ptf1a+/Cre(ex1);Notch2fl/fl mice, respectively (called N1ko and N2ko mice hereafter). These mice were born at the expected Mendelian ratio, and successful recombination of the floxed loci was confirmed by PCR (Fig. S1 A and B). N1ko mice have been previously described to show no major pancreatic abnormalities (16). Similarly, N2ko adult pancreata displayed no obvious morphological or functional abnormalities (Fig. S2). However, in mice older than 12 mo of age, we often noted a slight to moderate degree of focal exocrine atrophy with adipose tissue accumulation.
To study the role of Notch1 and Notch2 during pancreatic carcinogenesis, we crossed N1ko and N2ko mice with Kras mice for generation of Kras;N1ko and Kras;N2ko mice, respectively. Notably, Kras;N2ko mice showed no PanIN progression over time, whereas Kras and Kras;N1ko mice developed higher grade PanIN lesions, suggesting that Notch2 is involved in PanIN progression (Fig. 1 D and E). PanIN lesions from all genotypes expressed typical markers such as CK19 and MUC5AC and, somewhat surprisingly, HES1 (Tables S1–S3).
Development of MCN-Like Lesions in Kras;N2ko Mice.
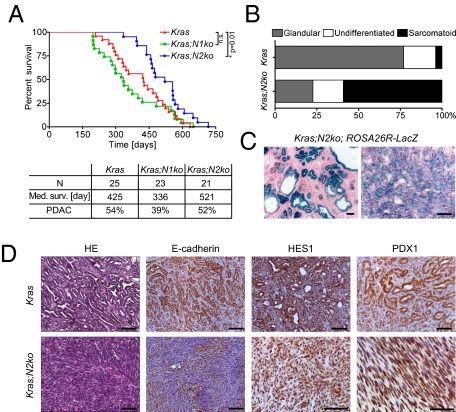
Frequently, albeit not in all mice, Kras;N2ko mice developed moderate to very large multilocular cysts. These cysts most often developed in the splenic part of the pancreas and showed a mucinous columnar epithelium resembling human MCN (Fig. S3 A and B). Rarely, goblet cells, high-grade dysplasia, and invasion into the adjacent stroma were noted. To characterize these lesions further, various markers, including those found in human MCNs, were analyzed. The cystic epithelial cells expressed PDX1, MUC5AC, and HES1, thus showing similar characteristics as PanIN lesions (Table S3). Consistent with the observation of an MCN-like preneoplastic lesion, we found an ovarian-like stroma surrounding the cystic lesions with estrogen receptor (ER)-positive and progesterone receptor-positive nuclei characteristic for human MCNs (19) (Fig. S3B and Table S7). To see whether the MCN-like lesions were derived from Notch2-deficient cells, cell lineage analysis was performed by crossing the Rosa26R+/LSL-lacZ reporter strain to Kras;N2ko mice. Indeed, we found all PanIN and MCN lesions to be X-Gal+ (Fig. 2C).
Fig. 2.
Deficiency of Notch2 prolongs survival and delays development of anaplastic PDAC. (A) Kaplan–Meier survival data and PDAC development of Kras, Kras;N1ko, and Kras;N2ko mice. Kras;N2ko mice have significantly prolonged survival compared with Kras and Kras;N1ko mice (P < 0.02). n.s., not significant. (B) Tumor differentiation analysis reveals more anaplastic PDAC in Kras;N2ko mice compared with Kras mice. (C) Positive X-Gal staining shows Cre-induced recombination in cells of MCN-like cysts and anaplastic PDAC in Kras;N2ko;Rosa26R+/LSL-lacZ mice. (D) Histological and immunohistochemical analysis of Kras and Kras;N2ko tumors. Expression of E-cadherin in Kras PDAC and low to absent expression in Kras;N2ko tumors. The Notch targets HES1 and PDX1 are expressed in tumors derived from both genotypes. (Scale bars: 50 μm.)
Distinct Roles for Notch1 and Notch2 During Tumor Development.
For analysis of PDAC development, a cohort of mice was followed for signs of disease progression or death. Kras and Kras;N1ko mice developed PDAC with similar characteristics regarding age of tumor development, tumor differentiation, rate, and sites of metastasis (Tables S4–S6). Kras;N1ko mice showed a slight, albeit not significant, reduction in median survival compared with Kras mice, supporting a nononcogenic role of Notch1 in KrasG12D-driven pancreatic carcinogenesis (Fig. 2A). However, in Kras;N2ko mice, a largely altered carcinogenic process was notable. These mice survived significantly longer than Kras and Kras;N1ko mice and only very rarely developed PDAC with ductal differentiation. Instead, Kras;N2ko mice either died without development of PDAC or developed highly aggressive anaplastic PDAC at a very advanced age (Fig. 2 A and B and Tables S4–S6). Histologically, most of these tumors were very large, showing a sarcomatoid cell pattern with a high proliferative index. Although we observed tumor areas that displayed features of poorly differentiated PDAC, we practically never observed G1/2 grades. Anaplastic PDAC showed an absence or low expression of E-cadherin and expressed PDX1, indicating its pancreatic origin (Fig. 2D). Lineage tracing showed PanIN and anaplastic PDAC development from Notch2-ablated pancreatic cells (Fig. 2C). Surprisingly, as was seen in MCN-like lesions, many cells expressed HES1, suggesting Notch2-independent regulation (Fig. 2D). Kras;N1ko and Kras;N2ko PDAC showed an absence of the respective Notch receptor, whereas expression was notable in Kras cancer cells (Figs. S1D and S4). To determine whether deficiency of Notch2 led to up-regulation of other Notch receptors, we tested Kras and Kras;N2ko PDAC cells for expression of Notch1–4. Here, we did not detect a consistent compensatory expression pattern of other Notch receptors in Kras;N2ko mice (Fig. S4).
Molecular Analysis of Key Signaling Pathways in Notch2-Deficient PDAC.
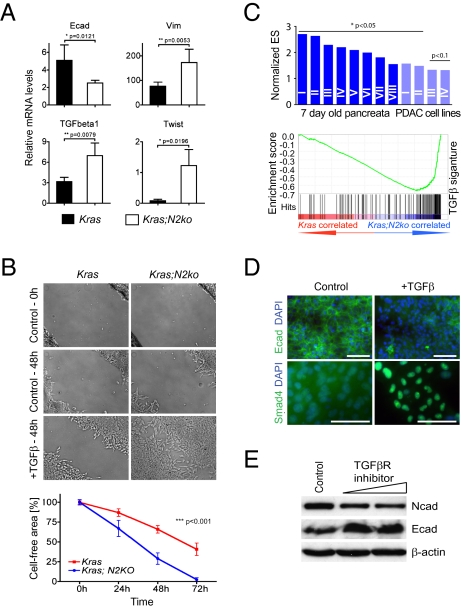
Analysis of genetic alterations typically found in PDAC showed no differences in p16Ink4a, p19Arf, p53, and Smad4 status between low-passage cancer cells isolated from Kras and Kras;N2ko PDAC (Tables S8 and S9). Consistent with low E-cadherin expression, we found increased levels of Twist, Snail, Slug, vimentin, and TGF-β1 in Kras;N2ko cancer cells, suggesting a high rate of epithelial-to-mesenchymal transition (EMT) (Fig. 3A). Because EMT has been associated with TGF-β signaling, we next tested integration of the pathway. Using a wound-healing assay, we found significantly increased cell migration of Notch2-deficient cancer cells (Fig. 3B). Gene set enrichment analysis (GSEA) was performed using pancreatic tissue at 7 d of age and cancer cells isolated from Kras and Kras;N2ko PDAC, as previously described (9), and revealed significant enrichment of several TGF-β signatures in Kras;N2ko preneoplastic tissue and cancer cells (Fig. 3C and Tables S10 and S11). Next, expression of E- and N-cadherin was studied in the presence of a TGF-β receptor inhibitor. Here, we found a reversed EMT process with increased expression of E-cadherin and down-regulation of N-cadherin (Fig. 3E), whereas addition of TGF-β led to down-regulation of E-cadherin and translocation of SMAD4 to the nucleus (Fig. 3D). These results suggest that TGF-β signaling is increased in Kras;N2ko PDAC yet responsive to either inhibition or activation in the absence of Notch2.
Fig. 3.
EMT is a prominent feature in Kras;N2ko PDAC. (A) Quantitative RT-PCR analysis of EMT-associated genes expressed by cancer cells from Kras and Kras;N2ko PDAC (n = 4 for each genotype). (B) Assessment of cell migration in wound closure assays performed in Kras and Kras;N2ko cells treated with TGF-β. Wound closure is delayed in Kras cells compared with Kras;N2ko cells. Quantification of wound closure is plotted as the percentage of the cell-free area over time. (C) Comparison of TGF-β gene sets by GSEA reveals significantly up-regulated TGF-β signatures in Kras;N2ko pancreata isolated from 7-d-old mice (dark blue, n = 2 and 4) and cancer cells (light blue, n = 6 each). A positive normalized enrichment score indicates elevated TFG-β–associated gene expression. Roman numbers refer to the detailed analysis in Tables S10 and S11. (D) Kras;N2ko cells reveal morphological and molecular responses characteristic of EMT in response to TGF-β, including loss of E-cadherin expression and nuclear translocation of SMAD4. (Scale bars: 50 μm.) (E) Treatment with the TGF-β receptor inhibitor SB431542 is sufficient to reverse the EMT-associated cadherin switch, suggesting that EMT in Kras;N2ko cells is dependent on a TGF-β autocrine loop.
Deficiency of Notch2 Modulates Myc Signaling.
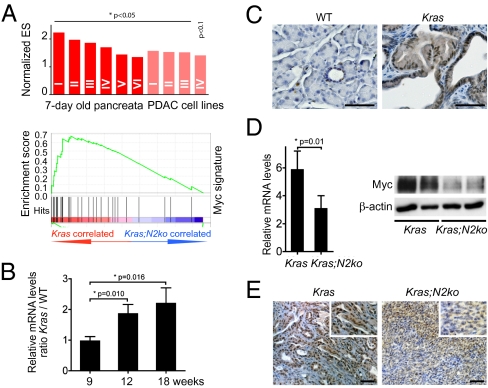
To elucidate the oncogenic role of Notch2 further, we screened Kras and Kras;N2ko preneoplastic pancreatic tissue and cancer cells using GSEA. Here, we noted highly significant enrichment of several Myc signatures, suggesting that Notch2 modulates Myc signaling (Fig. 4A and Tables S12 and S13). Compatible with deregulation of Myc signaling during early carcinogenesis, we found increased Myc expression in PanIN lesions as well as increasing mRNA levels in KrasG12D-induced pancreatic tissue during preneoplastic progression (Fig. 4 B and C and Tables S1–S3). We next examined Kras and Kras;N2ko cancer cells and found reduced mRNA and, most importantly, reduced protein levels in Kras;N2ko cells (Fig. 4D). Immunohistochemistry of Myc in PDAC of Kras mice and anaplastic PDAC of Kras;N2ko mice revealed a heterogeneous yet decreased expression pattern in Kras;N2ko mice (Fig. 4E and Tables S1–S3), suggesting that Myc protein expression is indeed down-regulated in Notch2-ablated preneoplastic and malignant pancreatic cells.
Fig. 4.
Myc is up-regulated during pancreatic carcinogenesis and down-regulated in Kras;N2ko mice. (A) GSEA shows significantly enriched Myc signatures in Kras vs. Kras;N2ko pancreata isolated from 7-d-old mice (dark red, n = 2 and 4) and primary cancer cells (light red, n = 6 each). Roman numbers refer to detailed analysis in Tables S12 and S13. (B) Myc transcript levels increase during carcinogenesis in Kras pancreata at indicated time points. Values represent WT-to-Kras ratio of relative expression levels (n = 3 for each time point). (C) Expression of Myc is low in the normal pancreas and increases in PanIN lesions of Kras mice. (D) Kras;N2ko cancer cells (n = 4) show decreased Myc mRNA and protein expression compared with Kras cells (n = 5). (E) Immunohistochemical staining in Kras;N2ko-derived anaplastic PDAC shows lower expression of Myc compared with Kras PDAC. (Scale bar: 50 μm.)
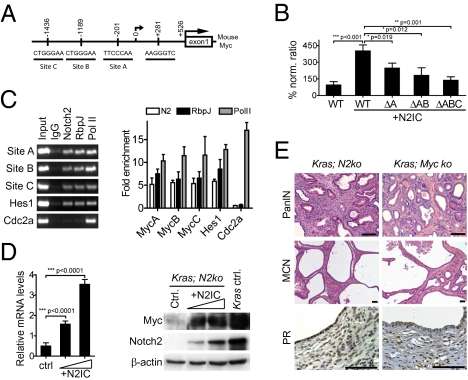
Recently, several Notch/Rbpj binding sites in the murine Myc promoter have been described (20). To analyze transcriptional regulation of Myc further, we considered three Notch/Rbpj signaling binding sites of interest in the Myc promoter (Fig. 5A). To test the relevance of each binding site, we transfected Kras;N2ko cancer cells with activated Notch2 (N2IC) and luciferase reporter vectors with one, two, or all three Notch/Rbpj sites mutated. As shown in Fig. 5B, all three sites seemed to be functional for transcriptional regulation. Intriguingly, we found Myc promoter induction through Notch2 in every cell line tested. We next performed ChIP to substantiate the reporter assay results in Kras cancer cells. ChIP demonstrated Notch2 and RbpJ binding to the Myc promoter. In fact, the increased Myc promoter occupation by Notch2 and RbpJ was comparable to that of Notch2 binding to the Hes1 promoter (Fig. 5C). Intriguingly, a similar result was obtained in the human PDAC cell lines MiaPaCa2 and Panc1, in which two Notch/Rbpj binding sites are conserved between humans and mice (Fig. S5). We next tested whether N2IC would increase Myc expression in Kras;N2ko and Panc1 cells. As shown in Fig. 5D, Myc mRNA and protein expression was increased in N2IC-transfected cells, suggesting transcriptional regulation.
Fig. 5.
Myc is a downstream target of Notch, and its ablation resembles features of the Notch2-deficient phenotype. (A) Analysis of Notch/Rbpj binding sites in the mouse Myc promoter using the consensus RTGGGAA motif reveals three sites: A, B, and C. (B) Activity of a Myc promoter fragment containing binding regions A, B, and C was analyzed using luciferase reporter assays. Kras;N2ko cells were cotransfected with Myc luciferase plasmids and N2IC. Mutations in the respective binding sites decrease activation of Myc. Activities were corrected for transfection efficiency by normalizing with Renilla luciferase activity and are expressed as a percentage of induction. (C) ChIP analyses using the indicated antibodies were analyzed by PCR for sites of interests. Products of the exponential phase of PCR are shown. Hes1 promoter primer served as positive control, and Cdc2a promoter primers as negative control. Quantitative PCR indicates that Notch2 binds to regions A, B, and C of the Myc promoter comparable to a binding site in the Hes1 promoter. (D) Transfection of N2IC stimulates Myc expression in Kras;N2ko cells in a dose-dependent manner. Notch2 and Myc expression levels of Kras control are shown for comparison. (E) Myc and Notch2 ablation in Kras mice results in similar phenotypes. Kras;N2ko and Kras;Myc-ko mice develop PanIN1 but not advanced PanIN2/3 lesions and MCN-like lesions with progesterone receptor-positive (PR+) surrounding stroma. Brightness and contrast levels were adjusted across the whole image for each panel. (Scale bar: 50 μm.)
To analyze Myc signaling in pancreatic carcinogenesis in vivo, we interbred previously described Mycfl/fl mice (21) with Pdx1-Cre;Kras+/LSL-G12D mice to obtain Myc-ablated Kras mice. Although breeding was hindered by exocrine atrophy occurring in most animals, we could analyze two mice 11 and 12 mo of age that showed a phenotype of only PanIN1 but not higher grade lesions, strongly supporting our hypothesis of Myc signaling being essential for PanIN progression. Additionally, we observed the development of MCN-like lesions with ovarian-like stroma, similar to Kras;N2ko mice (Fig. 5E).
Discussion
Notch Signaling Activation in Pancreatic Carcinogenesis.
In this study, we have evaluated the role of the Notch receptors 1 and 2 in pancreatic carcinogenesis in vivo using the well-established conditional KrasG12D model generated by Tuveson and co-workers (3). Although inhibition of PanIN progression in Kras;N2ko mice goes along with the results of inhibition of Notch signaling through γ-secretase inhibitor treatment (10), some differences between the models are notable. Plentz et al. (10) found a high relative increase of Notch3 mRNA in duct cells derived from PanIN-bearing pancreata and cells isolated from PDAC. Although we also found an increase in expression of Notch3 in PanIN-bearing compared with WT pancreata, expression was low compared with Notch1 and Notch2 levels. Reasons may include use of different mouse models as well as analysis of different tissue samples. In cancer cells isolated from PDAC of Kras mice, however, we also found much lower mRNA and protein levels of Notch3 compared with Notch2. In fact, Notch2 was by far the most prominently expressed Notch receptor during PanIN development and in PDAC, a finding supported by earlier studies (5). Importantly, we found no consistent up-regulation of any other Notch receptor in Notch2-deficient PDAC cells, suggesting that these cells could not easily reconstitute loss of Notch2 by any other Notch receptor. Interestingly, we did not observe loss of HES1 expression in either Notch1- or Notch2-ablated pancreata, suggesting that Hes1 may be regulated by other signaling pathways, as suggested previously (12, 16).
Although the downstream signaling of different Notch receptors and ligand specificity are complex, the differential pancreatic expression of Notch1 and Notch2 is noteworthy. The predominant expression of Notch1 in acinar cells goes along with our previous result of impaired regeneration in conditional Notch1-deficient mice during acute pancreatitis (16). Interestingly, Murtaugh and co-workers (11) found Notch1-activated mature acinar cells to be susceptible to PanIN initiation and progression. The hypothesis of acinar cells as potential cells of origin for PDAC has recently gained much interest because of the plasticity of this cell type, its potential for initiation of preneoplastic lesions (22–25), and the involvement of Notch signaling (5, 11). Although Notch1 is expressed in the acinar compartment, expression was absent in PanIN lesions when analyzed using transgenic Notch1-GFP reporter mice. Along this line, we did not observe fewer PanINs when Notch1 was ablated in our model. Instead survival and tumor incidence was reduced, although this finding was not significant. Of note, Notch1 ablation in Pdx1-Cre;KrasG12D mice was recently shown to result in increased PanIN progression, supporting the concept that Notch1 has no oncogenic role in pancreatic carcinogenesis (26).
Expression of Notch2 in ductal cells has been described previously and increases in metaplastic ductal cells (27, 28). Recently, centroacinar cells were described to show features of progenitor cells, including respective marker expression, sphere formation ability, and differentiation into different pancreatic lineages (15). These and our results suggest that a potential progenitor compartment in small ducts such as centroacinar cells expresses Notch2, a hypothesis supported by our expression studies using Notch2+/lacZ reporter mice. Because we observed PanIN1 initiation but no higher grade PanINs in Kras;N2ko mice, activation of Notch2 may be required for progression of PanIN lesions. However, other explanations remain possible. Because PanIN1 lesions are often encountered in pancreata of elderly people, it is possible that PanIN1 lesions may not actually precede PanIN2 and PanIN3 lesions but are mainly default lesions that may form from different pancreatic cells, including the acinar compartment. Consistent with this hypothesis is the induction of PanIN lesions but usually no development of invasive PDAC from acinar cells in Ela-Cre-ER;KrasG12D mice. Although our study did not directly address this intriguing question, it remains possible that PanIN1 lesions may originate from acinar cells, whereas initiation or progression of PanIN2/3 lesions may require a Notch-regulated potential progenitor compartment or an additional stimulus such as ongoing inflammation (25, 29).
Development of MCN-Like Lesions and Anaplastic PDAC in Kras;N2ko Mice.
The blockade of PanIN progression and PDAC development in Notch2-deficient KrasG12D mice goes along with the longer survival of these mice. Eventually, these mice develop large cysts resembling MCNs and succumb from either pancreatic insufficiency or from the development of anaplastic PDAC. Development of MCN-like lesions may thus be a bypass route for pancreatic cells undergoing oncogenic stress. However, two scenarios are possible with either (i) a common cell of origin for PanIN and MCN development, in which the route to higher grade PanINs is blocked by Notch2 deficiency, or (ii) different cells of origin for each lesion type that respond differentially to KrasG12D in the presence or absence of Notch2.
Interestingly, an association of anaplastic PDAC and MCN has been repeatedly described in patients (30). However, we do not have enough evidence to conclude that MCNs are the direct precursors for PDAC in Kras;N2ko mice. Further analysis is required to understand the cellular and molecular cues in Notch2-deficient malignant transformation. However, the clinical and experimental observations of the combined occurrence of MCN and anaplastic PDAC highlight the potential predictive capability of genotype-phenotype correlations in complex cancer mouse models.
TGF-β Signaling and EMT in Notch2-Deficient PDAC.
Molecular characterization of the anaplastic PDAC in Kras;N2ko mice showed evidence of EMT. Several reports have described an activating role of increased Notch signaling in EMT by regulation of E-cadherin repressors such as Snail or interaction with TGF-β signaling (31–34). TGF-β is known to play an ambivalent role in cancer biology. In the pancreas, conditional inactivation of TGF-β receptor 2 led to accelerated development and progression of well-differentiated PDAC (35). The development of late-occurring anaplastic PDAC with increased EMT is compatible with the dual role of TGF-β signaling in epithelial tumorigenesis. The effect of TGF-β receptor inhibition on E- and N-cadherin expression and exogenous TGF-β–induced nuclear translocation of SMAD4 suggest an intact TGF-β signaling axis. Indirect regulation of TGF-β may occur through deregulated Myc signaling, which is known to suppress the activation of TGF-β–induced genes such as p21CIP1, which has been shown to interact with Notch in various organs (36, 37). However, we could not detect consistent differences in p21CIP1 expression or related signatures between Kras and Kras;N2ko tumors.
Myc Signaling Is Regulated by Notch2 in PDAC.
Decreased Myc signaling in Kras;N2ko mice supports the hypothesis of Notch2-dependent Myc signaling as a key regulator of the carcinogenic process in the pancreas. Deregulation of Myc in PDAC has been described in many studies, and amplification occurs in about 30% of human PDAC as well as in murine PDAC (38–40). In recent studies, Myc signaling has been identified to play a key role in cell cycle regulation of PDAC cells (41, 42). Although these studies demonstrate the importance of deregulated Myc signaling in PDAC, our results suggest an early role during PanIN progression supported by early Myc amplification in precursor lesions (38). In a recent quantitative proteomic screen of preneoplastic PanIN lesions, Myc expression was identified in PanIN3 lesions (43).
We and others have previously characterized the important role of Myc in progenitor and acinar cell proliferation during development and adult homeostasis (21, 44, 45). Consistently, we found increased Myc expression throughout PanIN development in Kras mice. It is tempting to speculate that Myc and Ras signaling cooperatively promote tumor progression in a setting of active Notch. Notch signaling has been reported to cooperate with Ras, and several studies have reported direct transcriptional regulation of Myc by Notch1 (20, 46–48). Our finding that active Notch2 induces Myc expression in PDAC cells supports these reports. Although preliminary, the phenotypical similarities of Notch2 and Myc-ablated KrasG12D-induced pancreata with development of cystic lesions and a PanIN progression stop strongly support this hypothesis. Of consideration is the use of different Cre mice, Ptf1a+/Cre(ex1) and Pdx1-Cre mice, in Kras;N2ko and Kras;Myc-ko mice, respectively, because of extensive exocrine hypoplasia and early postnatal death of Ptf1a+/Cre(ex1);Mycfl/fl mice (21). Although we cannot rule out different target compartments in both Cre lines, this seems unlikely, given the similar phenotype in KrasG12D-activated mice (3).
The results from luciferase reporter and ChIP assays suggest that all three reported Notch/Rbpj binding sites in the Myc promoter are relevant for transcriptional regulation of Myc. On the basis of our findings, we report that Myc is regulated by Notch2. Why Notch1 ablation did not lead to similar alterations in early tumor progression in our model is not clear. A possible explanation would be a context- and cell-specific role of Myc and its regulation through Notch. A possible scenario may thus be that a progenitor cell (e.g., within the centroacinar compartment) is the target cell for cooperative Myc-Ras–induced tumor development propagated by Notch2 activation. The success of Notch inhibition as a chemopreventive approach to inhibit PanIN progression has been shown (10). This outcome is supported by our results. Of note, the same group has reported Myc amplification in KrasG12D-driven PDAC mouse models, adding evidence for a key role of this signaling pathway during the carcinogenic process (40). It will be of great interest to study the integration of the transcriptional programs regulated by Myc and Notch signaling in further detail, which may eventually help to explain the permissive signals regulating pancreatic plasticity and malignant transformation.
In summary, our results provide evidence for an essential role of Notch2 and Myc in the initiation of a neoplastic transformation program in pancreatic cells, whereas Notch1 has no oncogenic role, supporting the concept of distinctive roles of individual Notch receptors in cancer development. In addition, the data demonstrate the integrative interaction of regulators of cell fate and cell cycle signaling, thereby enhancing our biological understanding for unique approaches in this still untreatable disease.
Materials and Methods
Mouse Strains.
Kras+/LSL-G12D, Notch1fl/fl, Notch2fl/fl, Mycfl/fl, Ptf1a+/Cre(ex1), Pdx1-Cre, and Rosa26+/LSL-lacZ mice have been described before (3, 9, 17, 21). All experiments were performed according to the guidelines of the local animal use and care committees.
Detailed descriptions of additional procedures, including protein and mRNA analysis, immunohistochemistry, microarray/GSEA, luciferase-based reporter assays, and ChIP, are provided in SI Text.
Supplementary Material
Acknowledgments
We thank W. Gao (Genentech, Inc., CA), Y. Hamada (National Institute for Basic Biology, Okazaki, Japan), and C. A. Klug (University of Alabama, Birmingham, AB) for the generous gift of Notch reporter mice and A. Klinakis (Biochemical Research Foundation, Athens, Greece) for Myc plasmids. We are grateful to T. Sudo (Toray Industries Inc., Kamakura, Japan) for HES1 and to C. V. Wright (Vanderbilt University Medical Center, Nashville, TN) for PDX1 antibodies. We thank M. Neuhofer, S. Ruberg, and C. Köhler for excellent technical assistance. This work was supported by grants from the German Cancer Aid (Grant 107195), German Federal Ministry of Education and Research (Grant 01GS08115), Lustgarten Foundation (RFP05-14 and 06-12), and German Research Foundation (Grant SI 1549/1-1).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002423107/-/DCSupplemental.
References
- 1.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 2.Hruban RH, Iacobuzio-Donahue C, Wilentz RE, Goggins M, Kern SE. Molecular pathology of pancreatic cancer. Cancer J. 2001;7:251–258. [PubMed] [Google Scholar]
- 3.Hingorani SR, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 4.Pasca di Magliano M, et al. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev. 2006;20:3161–3173. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyamoto Y, et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 6.Pasca di Magliano M, et al. Common activation of canonical Wnt signaling in pancreatic adenocarcinoma. PLoS ONE. 2007;2:e1155. doi: 10.1371/journal.pone.0001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thayer SP, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanger BZ, et al. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 2005;8:185–195. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Siveke JT, et al. Concomitant pancreatic activation of Kras(G12D) and Tgfa results in cystic papillary neoplasms reminiscent of human IPMN. Cancer Cell. 2007;12:266–279. doi: 10.1016/j.ccr.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Plentz R, et al. Inhibition of gamma-secretase activity inhibits tumor progression in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology. 2009;136:1741–1749. doi: 10.1053/j.gastro.2009.01.008. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De La O JP, et al. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci USA. 2008;105:18907–18912. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakhai H, et al. Conditional ablation of Notch signaling in pancreatic development. Development. 2008;135:2757–2765. doi: 10.1242/dev.013722. [DOI] [PubMed] [Google Scholar]
- 13.Lewis AK, Frantz GD, Carpenter DA, de Sauvage FJ, Gao WQ. Distinct expression patterns of notch family receptors and ligands during development of the mammalian inner ear. Mech Dev. 1998;78:159–163. doi: 10.1016/s0925-4773(98)00165-8. [DOI] [PubMed] [Google Scholar]
- 14.Hamada Y, et al. Mutation in ankyrin repeats of the mouse Notch2 gene induces early embryonic lethality. Development. 1999;126:3415–3424. doi: 10.1242/dev.126.15.3415. [DOI] [PubMed] [Google Scholar]
- 15.Rovira M, et al. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci USA. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siveke JT, et al. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology. 2008;134:544–555. doi: 10.1053/j.gastro.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Besseyrias V, et al. Hierarchy of Notch-Delta interactions promoting T cell lineage commitment and maturation. J Exp Med. 2007;204:331–343. doi: 10.1084/jem.20061442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakhai H, et al. Ptf1a is essential for the differentiation of GABAergic and glycinergic amacrine cells and horizontal cells in the mouse retina. Development. 2007;134:1151–1160. doi: 10.1242/dev.02781. [DOI] [PubMed] [Google Scholar]
- 19.Zamboni G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410–422. doi: 10.1097/00000478-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Klinakis A, et al. Myc is a Notch1 transcriptional target and a requisite for Notch1-induced mammary tumorigenesis in mice. Proc Natl Acad Sci USA. 2006;103:9262–9267. doi: 10.1073/pnas.0603371103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakhai H, Siveke JT, Mendoza-Torres L, Schmid RM. Conditional inactivation of Myc impairs development of the exocrine pancreas. Development. 2008;135:3191–3196. doi: 10.1242/dev.017137. [DOI] [PubMed] [Google Scholar]
- 22.Habbe N, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci USA. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji B, et al. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137:1072–1082. doi: 10.1053/j.gastro.2009.05.052. 1082.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171:263–273. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerra C, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Hanlon L, et al. Notch1 functions as a tumor suppressor in a model of K-ras-induced pancreatic ductal adenocarcinoma. Cancer Res. 2010;70:4280–4286. doi: 10.1158/0008-5472.CAN-09-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rooman I, et al. Expression of the Notch signaling pathway and effect on exocrine cell proliferation in adult rat pancreas. Am J Pathol. 2006;169:1206–1214. doi: 10.2353/ajpath.2006.050926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KM, Yasuda H, Hollingsworth MA, Ouellette MM. Notch 2-positive progenitors with the intrinsic ability to give rise to pancreatic ductal cells. Lab Invest. 2005;85:1003–1012. doi: 10.1038/labinvest.3700298. [DOI] [PubMed] [Google Scholar]
- 29.Morris JP, IV, Cano DA, Sekine S, Wang SC, Hebrok M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010;120:508–520. doi: 10.1172/JCI40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan ZG, Wang B. Anaplastic carcinoma of the pancreas associated with a mucinous cystic adenocarcinoma. A case report and review of the literature. JOP. 2007;8:775–782. [PubMed] [Google Scholar]
- 31.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400–2407. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timmerman LA, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zavadil J, Cermak L, Soto-Nieves N, Böttinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23:1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ijichi H, et al. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006;20:3147–3160. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu M, et al. p21CIP1 attenuates Ras- and c-Myc-dependent breast tumor epithelial mesenchymal transition and cancer stem cell-like gene expression in vivo. Proc Natl Acad Sci USA. 2009;106:19035–19039. doi: 10.1073/pnas.0910009106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rangarajan A, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schleger C, Verbeke C, Hildenbrand R, Zentgraf H, Bleyl U. c-MYC activation in primary and metastatic ductal adenocarcinoma of the pancreas: Incidence, mechanisms, and clinical significance. Mod Pathol. 2002;15:462–469. doi: 10.1038/modpathol.3880547. [DOI] [PubMed] [Google Scholar]
- 39.Schreiner B, et al. Murine pancreatic tumor cell line TD2 bears the characteristic pattern of genetic changes with two independently amplified gene loci. Oncogene. 2003;22:6802–6809. doi: 10.1038/sj.onc.1206836. [DOI] [PubMed] [Google Scholar]
- 40.Bardeesy N, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci USA. 2006;103:5947–5952. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konig A, et al. NFAT-induced histone acetylation relay switch promotes c-myc-dependent growth in pancreatic cancer cells. Gastroenterology. 2010;138:1189–1199. doi: 10.1053/j.gastro.2009.10.045. .e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schild C, et al. PI3K signaling maintains c-myc expression to regulate transcription of E2F1 in pancreatic cancer cells. Mol Carcinog. 2009;48:1149–1158. doi: 10.1002/mc.20569. [DOI] [PubMed] [Google Scholar]
- 43.Pan S, et al. Quantitative proteomics investigation of pancreatic intraepithelial neoplasia. Electrophoresis. 2009;30:1132–1144. doi: 10.1002/elps.200800752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonal C, et al. Pancreatic inactivation of c-Myc decreases acinar mass and transdifferentiates acinar cells into adipocytes in mice. Gastroenterology. 2009;136:309–319.e9. doi: 10.1053/j.gastro.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Strom A, et al. Unique mechanisms of growth regulation and tumor suppression upon Apc inactivation in the pancreas. Development. 2007;134:2719–2725. doi: 10.1242/dev.02875. [DOI] [PubMed] [Google Scholar]
- 46.Palomero T, et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc Natl Acad Sci USA. 2006;103:18261–18266. doi: 10.1073/pnas.0606108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma VM, et al. Notch1 contributes to mouse T-cell leukemia by directly inducing the expression of c-myc. Mol Cell Biol. 2006;26:8022–8031. doi: 10.1128/MCB.01091-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weng AP, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.