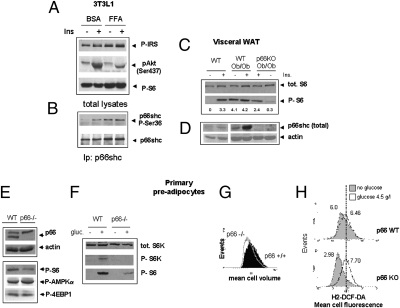
Fig. 4.
Involvement of p66shc in nutrient signaling through mTOR/S6K. (A and B) Immunoblot analysis of signaling events relevant to insulin responses in 3T3L1 cells exposed to excess FFA (oleic + linoleic + palmitic acid) for 16 h. Phosphorylation of Akt, p66shc, S6 (serine 235–236), and IRS-1 (Ser-636–639 and 307) were monitored. Image representative of at least two independent experiments. (C and D) Immunodetection of the S6 kinase substrate S6 (phospho-Ser and total), of p66shc and of actin as a loading control in WAT homogenates from mice of the indicated genotypes. Samples are from single animals. Mice in lanes 2, 4, and 5 were stimulated with 10 U/kg insulin. The blot is representative of two/three independent experiments. Numbers indicate band intensities (OD values, background subtracted) for phospho-S6. (E) Immunoblot analysis showing expression of p66shc in freshly isolated (1 g/L glucose, 20% FCS) adipocyte precursor cells. The p66shc band, missing in p66KO-derived cells, is indicated. Reduced phosphorylation of S6, but unchanged phosphorylation of 4EBP1 and AMPK α in p66KO cells is also shown. (F) Reactivation of the S6 kinase–S6 pathway in nutrient-starved preadipocytes in response to glucose (60 min). (G) Flow cytometry analysis of cell volume in exponentially growing P66-deficient and -proficient cells (passage 1–3). (H) Generation of reactive oxygen species in glucose-refed adipocyte precursors of different p66 genotype. Serum and glucose-starved cells were refed with 2 g/L glucose for 2 h (unshaded curve) or left untreated (shaded curve). Fluorescence of cells loaded with the redox sensitive dye DCF-DA was analyzed by flow cytometry. Numbers are mean cell fluorescence intensities. The vertical line indicates overlapping peak positions for stimulated cell populations. Data representative of two independent experiments.