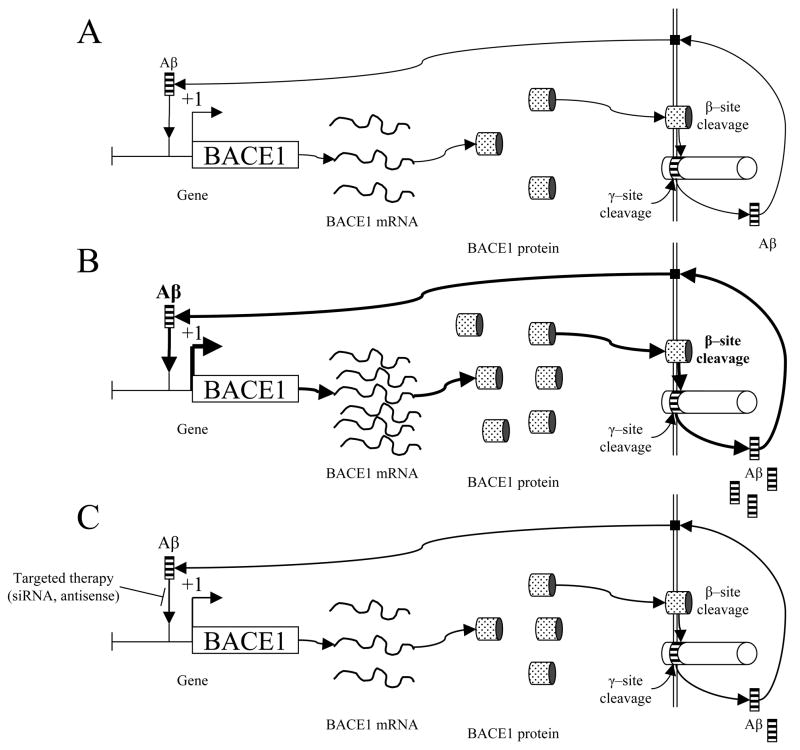
Fig. 1. Self–regulation of Aβ cleavage from AβPP through the BACE1 gene promoter and therapeutic implications.
A) Intranuclear Aβ peptide would function as a transcription factor, upregulating BACE1 gene transcription. This would stimulate production of BACE1 mRNA as a template for BACE1 protein production. BACE1 would cleave AβPP at the β–cleavage site. When this is followed by γ–cleavage, extracellular Aβ is released. Aβ would then enter the cell through currently–uncharacterized receptor(s) that could include FPR2, insulin receptor, or NMDA receptors (Verdier, et al., 2004). Once within the cell, Aβ would then be transported into the nucleus to renew the cycle. B) Under pathogenic conditions, Aβ levels would have been stimulated to increase BACE1 transcription to the extent that normal Aβ clearance pathways, such as IDE, would fall behind. Additional Aβ would be transported into the cell, to stimulate increased BACE1 transcription, resulting in an uncontrolled positive feedback loop. C) Therapeutic blockage of Aβ–BACE1 promoter interaction by sequence specific siRNA or antisense DNA oligomers would result in reduced BACE1 gene transcription, theoretically permitting Aβ clearance mechanisms to catch up to production. Reduced production would restore a state similar to pre disease levels.