Abstract
Melanoma cells are highly resistant to anoikis, a form of apoptosis induced in non-adherent/inappropriate adhesion conditions. Depleting B-RAF or the pro-survival Bcl-2 family protein Mcl-1 renders mutant B-RAF melanoma cells susceptible to anoikis. In this study, we examined the effect of targeting B-RAF on the survival of primary stage melanoma cells cultured in 3-D type I collagen gels, which partially mimics the dermal microenvironment. Depletion/inhibition of B-RAF with siRNA or the mutant B-RAF inhibitor, PLX4720, induced apoptosis of mutant B-RAF melanoma cells in 3-D collagen. Apoptosis was dependent on two up-regulated BH3-only proteins, Bim-EL and Bmf, and was inhibited by ectopic Mcl-1 expression. Akt3 activation has been associated with the survival of melanoma cells. Mutant B-RAF melanoma cells ectopically expressing a constitutively activated form of Akt3 or endogenously expressing mutant Akt3 were protected from apoptosis induced by B-RAF knockdown or PLX4720 treatment. Furthermore, intrinsically resistant metastatic melanoma cells displayed elevated Akt phosphorylation in 3-D collagen and were rendered susceptible to PLX4720 by Akt3 knockdown. Importantly, myristylated Akt3 prevented B-RAF targeting induced up-regulation of Bim-EL and Bmf in 3-D collagen and partially protected Mcl-1 depleted cells from apoptosis. These findings delineate how mutant B-RAF protects melanoma cells from apoptosis and provide insight into possible resistance mechanisms to B-RAF inhibitors.
Keywords: Anoikis, apoptosis, B-RAF, Bim-EL, Bmf, Mcl-1
Introduction
Melanoma is the deadliest form of skin cancer and its incidence is on the rise. It is treatable by surgical excision when detected at a non-invasive radial growth phase (RGP) stage. However during the vertical growth phase (VGP), it invades through the basement membrane and into the dermis. Two major issues thwarting the efficacy of melanoma treatments are the propensity of VGP cells to metastasize from the dermis and resistance to chemotherapeutic regimens.
A significant advance in understanding the biology of melanoma was the identification of B-RAF mutations in approximately 60% of these tumors (1). B-RAF is a serine-threonine kinase that activates the MEK-ERK1/2 pathway. The most frequent mutation in B-RAF is a valine to glutamate substitution at codon 600 (V600E), which results in B-RAF activation and occurs early in the disease. The downstream MEK-ERK1/2 pathway is activated in melanomas (2, 3) and is required for the proliferation, resistance to apoptosis and invasion of mutant B-RAF melanoma cells (4–6). These findings have focused efforts on the development of inhibitors to target B-RAF in melanoma. Recently, a structure-guided approach led to the generation of PLX4720, a drug which binds near the ATP-binding site in B-RAF and exhibits a 10-fold lower IC50 towards B-RAFV600E compared to wild-type B-RAF (7). In an early-stage clinical trial, PLX4032, a structural analog of PLX4720, showed strong anti-tumor effects in patients genotyped as harboring mutant B-RAF (8). However, the majority of patients administered PLX4032 exhibit a partial response. Also, B-RAF and MEK inhibitors in pre-clinical assays typically elicit a cytostatic response (4, 7, 9). These findings indicate that it will be critical to promote cytotoxic effects in B-RAF targeted tumors in order to obtain prolonged tumor regression in patients.
A crucial step in tumor progression is the acquisition of resistance to anoikis, a form of apoptosis induced by loss of adhesion or inappropriate adhesion to the extracellular matrix (ECM) (10). The ability of melanoma cells to counteract anoikis is critical as they move out from the epidermis, invade the type I collagen-rich dermis, survive in the circulatory system, and eventually establish metastatic colonies. We and others have shown that human melanocytes are susceptible to anoikis whereas mutant B-RAF harboring VGP melanoma cells are resistant (11, 12). B-RAF signaling is implicated in the resistance of VGP melanoma cells since B-RAF depletion or MEK inhibition renders invasive cells susceptible to anoikis (12, 13). These effects were associated with enhanced expression of the pro-apoptotic BH3-only protein, B-cell lymphoma 2 interacting mediator extra long isoform (Bim-EL), and decreased levels of the pro-survival protein, myeloid cell leukemia-1 (Mcl-1) (13, 14).
Integrin-mediated adhesion to the ECM component, fibronectin, protects B-RAF knockdown melanoma cells from undergoing apoptosis (12). Fibronectin is a minor component of the dermis (15) and is deposited at pre-metastatic niches (16). Furthermore, fibronectin binding integrins, αvβ3 and α4β1, are highly expressed in melanoma cells (17) and use of peptides to block cell binding to fibronectin prevents melanoma cell growth in mouse models (18). The mechanism of fibronectin-dependent protection from apoptosis is mediated, at least in part, through activation of Akt (12). Of the three Akt isoforms, Akt3 likely plays a key role since it is the predominately activated Akt isoform in melanoma cells and knockdown of Akt3 co-operates with B-RAF knockdown to promote apoptosis (19, 20).
It will be critical to understand how to maximize the therapeutic effects of targeting B-RAF. To this end, we utilized inhibitor and RNA interference approaches to target B-RAF in mutant B-RAF melanoma cells. Analysis was performed on RGP or VGP-like cells in 3-D collagen, to more accurately recapitulate the dermal microenvironment. We show that targeting B-RAF induces apoptosis in 3-D collagen that is controlled by levels of the BH3-only proteins, Bim-EL and Bcl-2-modifying factor (Bmf), and the pro-survival protein Mcl-1. Constitutive activation of Akt3 protects against B-RAF knockdown/inhibition, prevents the up-regulation of Bim-EL and Bmf, and partially protects melanoma cells from Mcl-1 depletion. Together, these findings delineate mutant B-RAF-dependent survival mechanisms in tumor cells and provide insight into possible modes of resistance to B-RAF inhibitors.
Material and Methods
Cell culture
Human WM793, WM35, WM278, WM46, and 1205Lu cells were generously provided by Dr. Meenhard Herlyn during the time period 2004–2009; A375 cells were purchased from ATCC. In all cases, early passage cultures were stored and used for the basis of these experiments. All cells were cultured in MCDB153 (Sigma-Aldrich, St Louis, MO) containing 20% Leibovitz L-15 medium, 2% fetal bovine serum and 5 μg/ml insulin, except A375 cells that were cultured in DMEM with 10% fetal bovine serum. WM793 and WM278 are VGP-like; WM35 display RGP properties; WM46, A375 and 1205Lu are metastatic lines.
Antibodies
The following antibodies were purchased from Cell Signaling Technology, (Beverley, MA): anti-pan Akt (#9227), anti-Akt3 (#4059), anti-phospho-Akt (Ser473, #9271), anti-phospho-(Ser/Thr) Akt substrate (#9611), anti-phospho-ERK (Thr202/Tyr204, #4377), anti-HA (#2367), anti-phospho-GSK3β (Ser9, #9336). Anti-Mcl-1 (#559027) and anti-GSK3β (#610201) were from BD Transduction (San Jose, CA). Anti-actin (# A2066) was from Sigma-Aldrich. Anti-Bim (#AAP-330) was from Stressgen (San Diego, CA).
Western blotting
Western blotting was performed as previously described (12). Briefly, cells were washed and lysed directly in Laemmli sample buffer. Lysates were separated on SDS polyacrylamide gels and transferred to polyvinylidene difluoride membranes. Proteins were detected using the indicated primary antibody and the signal was detected using peroxidase-conjugated secondary antibody followed by development using chemiluminescence substrate (Pierce, Rockford, IL). Chemiluminescence was detected using a Versadoc Imaging system (BioRad, Hercules, CA) and was quantitated using Quantity-One software.
siRNA transfections
WM793 cells were transfected with siRNAs using Oligofectamine (Invitrogen, Carlsbad, MA), as previously described (12). Non-targeting control, B-RAF #1, Mcl-1 #11, Bmf #7, and Akt3 siRNAs were purchased from Dharmacon (Lafayette, CO). Bim siRNA were from Cell Signaling Technology (13). All siRNAs were utilized at a final concentration of 25 nM. Cells were transfected for 72 h before subsequent treatment or analysis.
Generation of lentiviruses and cell lines
Wild-type Akt3 and N-terminal myristylated Akt3 signal were amplified from human Akt3 cDNA plasmid (Addgene, Cambridge MA) using KOD Hot Start DNA polymerase kit (Novagen, Darmstadt, Germany). The primer set for wild-type Akt3 was: forward: 5' caccatgtacgcctacgacgtgcccg 3' and reverse 5' ttattctcgtccacttgcagagtagg 3'. Myr-HA-AKT3 was amplified in the same way except that forward primer, 5' caccatggggagcagcaagag 3', was used in the PCR reaction. In both constructs, the HA epitope tag was retained. Akt fragments were then cloned into pENTR™/D-TOPO vector (Invitrogen) according to the manufacturer's guidelines. Entry plasmids were recombined with pLenti6/UbC/V5-DEST using the LR Clonase II kit and protocol (Invitrogen) to generate pLenti6/UbC/HA-AKT3 and pLenti6/UbC/Myr-HA-AKT3. Constructs were verified by DNA sequencing. Lentiviruses were generated as described before (13) using ViraPower™ Lentiviral Gateway™ Expression kit (Invitrogen). Cells were incubated for 72 h in the presence of lentiviral supernatant. Transduced cell populations were selected with 5 μg/ml blasticidin.
Wild type Mcl-1 cDNA sequence was PCR amplified from human Mcl-1 cDNA kindly provided by Dr. Steven Edwards (University of Liverpool, UK(21)) using KOD hot Start DNA polymerase kit and the primers 5' caccatgtttggcctcaaaagaaccg 3' and 5' ctatcttattagatatgcc 3'. DNA fragment was cloned into pENTR™/D-TOPO vector and the entry plasmid was then recombined with pLenti4/TO/V5-DEST to form destination plasmid pLenti4/TO/Mcl-1. WM793 cells that stably express tet repressor (WM793TR (13)) were infected with lentivirus for 72 h before selection with 25 ug/ml zeocin.
Three-dimensional (3-D) collagen gels
Collagen gels were cast by mixing the following on ice: Eagle's Minimum Essential Medium (Lonza, Inc. Walkersville, MD), 2 mM L-glutamine, 2% FBS, 0.15% sodium bicarbonate and 0.8 mg/ml bovine type-I collagen (Organogenesis Inc. Canton, MA). Cells were seeded in 2 ml collagen gels and incubated at 37°C for 30 min. After polymerization, the collagen gel lattice was overlaid with 2 ml medium.
Apoptosis assays
3-D collagen gels were dissolved in 1 mg/ml collagenase (Sigma-Aldrich, St Louis MO) solution to release cells. Adherent cells in 2-D cell cultures were trypsinized. Cells were washed once in PBS and resuspended in 100 μl binding buffer (10 mM HEPES, 140 mM sodium chloride, 2.5 mM calcium chloride) at a concentration of 106 cells/ml. Cells were then stained with 5 μl annexin V-APC (BD Biosciences) for 15 min before 400 μl binding buffer was added. Staining was measured by flow cytometry on the FACSCalibur (BD Biosciences) and data analyzed using Flowjo software (Three Star, Inc. Ashland, OR).
Quantitative RT-PCR
Total RNA was extracted from melanoma cells by using 5 Prime Perfect Pure RNA isolation kit (5 Prime, Inc. Gaithersburg, MD). RNA (1 μg) was reverse transcribed, and 1/20 of the resulting cDNA was used to detect mRNA abundance with primers for GAPDH (forward, 5' tggaccaccaactgcttag 3'; reverse, 5' gatgcagggatgatgttc 3'), Bim-EL (forward, 5' tccctgctgtctcgatcctc 3'; reverse, 5' gctcttcggctgcttggtaa 3') and Bmf (forward, 5' gaggtacagattgcccgaaag 3'; reverse, 5' ttcaaagcaaggttgtgca 3'). Reactions were performed in SYBR Green mix and analyzed using the MyiQ real-time PCR detection system (Bio-Rad). Relative mRNA levels were calculated using the comparative Ct (ΔCt) method. Quantitation of mRNA levels represents data from three independent experiments.
Results
Targeting B-RAF renders mutant B-RAF melanoma cells susceptible to apoptosis in 3-D collagen
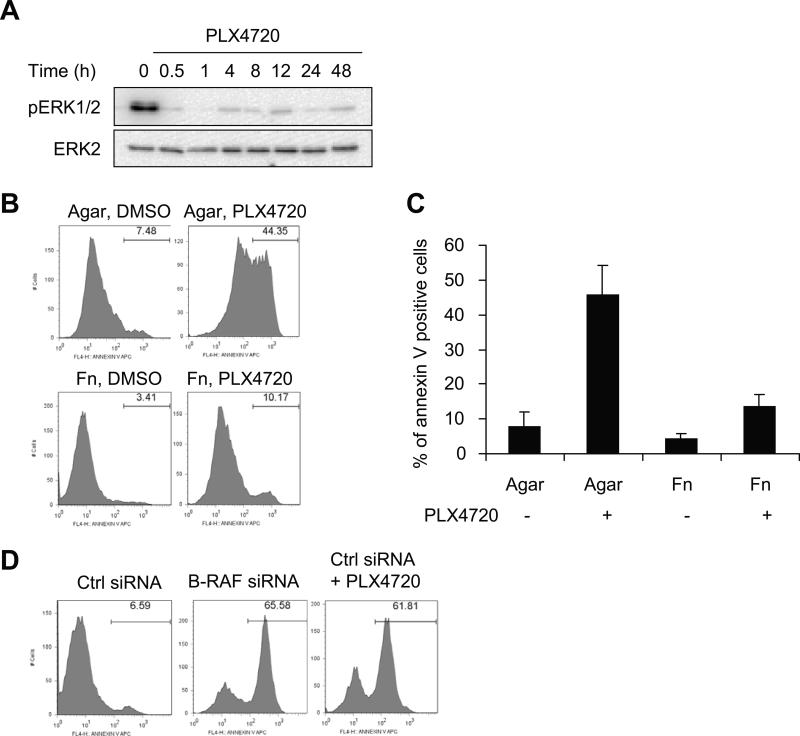
We have previously shown that B-RAF knockdown renders the B-RAFV600E melanoma WM793 cells susceptible to apoptosis in suspension (12). Here, we tested the mutant selective B-RAF inhibitor, PLX4720, in similar assays. The effectiveness of PLX4720 to inhibit B-RAF signaling in WM793 cells was manifested by its rapid and persistent inhibition of phosphoERK1/2 levels (Fig. 1A). Similar to our previous results with B-RAF knockdown, PLX4720 treatment increased annexin V staining in suspended but not in fibronectin adherent cells (Fig. 1B, quantitated in 1C). WM793 display properties of VGP and to more faithfully recapitulate signaling in the dermal microenvironment, we extended our studies to 3-D type-I collagen gels. WM793 cells transfected with control siRNAs and subsequently cultured in 3-D collagen displayed low levels of annexin V staining (Fig. 1D). By contrast, B-RAF knockdown or PLX4720-treated WM793 cells in 3-D collagen displayed enhanced annexin V staining. We have previously observed similar results between annexin V and cleaved caspase 3 staining indicating activation of the intrinsic apoptotic pathway (14). These data show that mutant B-RAF is required for protection of melanoma cells in suspension and 3-D collagen conditions. WM793 cells express collagen receptors including the α1 and α2 integrins (12) and α2β1 mediates cell arrest in fibrillar collagen gels in some melanoma lines (22). However, efficient knockdown of α1 and α2 integrins did not alter PLX4720-induced apoptosis in WM793 cells (Supplemental Fig. 1), indicating that these integrins are not required for the apoptosis mediated by B-RAF inhibition.
Figure 1.
PLX4720 treatment renders mutant B-RAF cells susceptible to apoptosis. A, WM793 cells were treated with 0.5 μM PLX4720 for the times indicated and cell lysates analyzed by Western blotting with phosphoERK1/2 and total ERK2 antibodies. B, WM793 cells were plated on agar or fibronectin in the absence/presence of 0.5 μM PLX4720. After 48 h, cells were harvested and stained with annexin V-APC for apoptosis analysis by flow cytometry. X axis, fluorescence intensity; Y axis, cell counts, with percent of annexin V-APC staining positive cells indicated in each condition. C, Quantitation of data from three independent experiments was represented by the mean percentage of cells staining positive for annexin V-APC. D, WM793 cells were transfected with control or B-RAF siRNA. Seventy-two hours post-transfection, cells were harvested, seeded in 3D-collagen gels. Cells were treated with 0.5 μM PLX4720 or DMSO (vehicle control). After 48 h, collagen gels were dissolved in collagenase solution and collected cells were analyzed by annexin V-APC staining and flow cytometry analysis.
Up-regulation of Bim-EL and Bmf contributes to apoptosis in 3-D collagen following B-RAF targeting
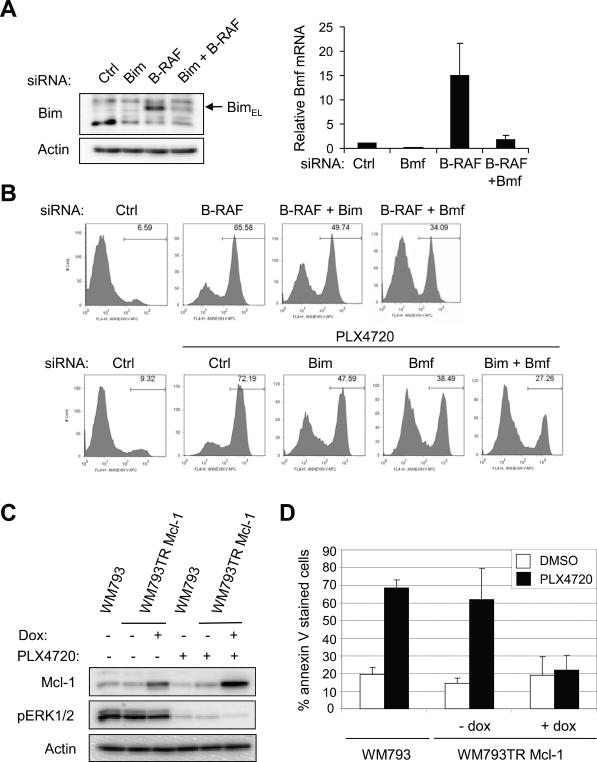
BH3-only proteins, such as Bim-EL and Bmf, are up-regulated following B-RAF knockdown or MEK inhibition in 2-D monolayer cultures of melanoma cells (13, 23–25). Initially, we validated that the up-regulation of Bim-EL and Bmf following B-RAF knockdown was efficiently prevented by siRNAs targeting either Bim or Bmf (Fig. 2A). Of note, we used qRT-PCR to detect Bmf due to the unavailability of a commercial antibody that detects a specific endogenous Bmf band, an observation consistent with Schmelzle et al (26). Next, we addressed the contribution of Bim-EL and Bmf to apoptosis in B-RAF-depleted cells in 3-D collagen. Bim-EL depletion partially prevented apoptosis in B-RAF-knockdown and PLX4720-treated WM793 cells in 3-D collagen (Fig. 2B). Similarly, Bmf depletion was effective at preventing apoptosis in B-RAF knockdown and PLX4720-treated cells. Furthermore, Bim-EL and Bmf co-knockdown was more effective than either individual knockdown at preventing apoptosis in PLX4720-treated cells (Fig. 2B). These data show that both Bim-EL and Bmf are required for apoptosis of B-RAF targeted cells in 3-D collagen.
Figure 2.
Apoptosis in 3-D collagen following B-RAF targeting is regulated by Bcl-2 family proteins, Bim-EL, Bmf and Mcl-1. A, WM793 cells were transfected with non-targeting (Ctrl), B-RAF, Bim and Bmf siRNAs, as indicated. After 72 h, cell lysates were analyzed by Western blotting for Bim-EL (left panel) or harvested for total RNA isolation and qRT-PCR analysis for Bmf (right panel). B, WM793 cells were transfected with control, B-RAF, Bim and Bmf siRNAs, as indicated, for 72 h. Transfected cells were cultured in 3-D collagen gels for 48 h in the absence/presence of 0.5 μM PLX4720 before analysis by flow cytometry for annexin V. C, WM793 and WM793TR Mcl-1 cells were treated −/+ 100 ng/ml doxycycline to regulate Mcl-1 expression and −/+ PLX4720, as indicated. Cell lysates were analyzed by Western blotting for Mcl-1, phosphoERK1/2 and actin. D, WM793 and WM793TR Mcl-1 cells were treated −/+ doxycycline for 3 days before being incorporated in 3-D collagen gels in serum-free conditions Cells in 3-D collagen were treated −/+ PLX4720 and after 48 h, processed for annexin V staining. The quantitation of mean −/+ SD from three independent experiments is shown.
Ectopic expression of Mcl-1 protects against PLX4720-induced apoptosis in 3-D
BH3-only proteins act, at least in part, by inhibiting pro-survival Bcl-2 proteins (27, 28). We have previously shown that B-RAF-MEK signaling enhanced protein stability of the pro-survival protein, Mcl-1 (14). To determine whether enhanced Mcl-1 expression was sufficient to confer resistance to apoptosis in B-RAF targeted cells, we engineered an inducible Mcl-1 expression cell line. Following doxycycline treatment, enhanced expression of Mcl-1 was detected in both non-treated and PLX4720-treated cells (Fig. 2C). Importantly, enhanced Mcl-1 expression protected WM793 cells from apoptosis induced by PLX4720 treatment in 3-D collagen (Fig. 2D). Mcl-1 also protected cells from apoptosis induced by MEK inhibition with U0126 (data not shown).
Constitutive activation of Akt3 protects against B-RAF inhibition
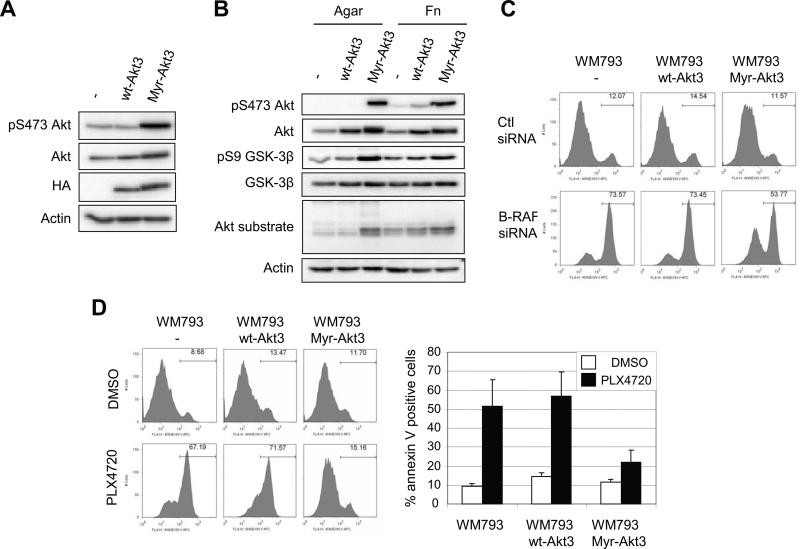
Adhesion to fibronectin, but not to collagen, enhances levels of phospho-Akt in WM793 cells (12) and Akt3 is the main Akt isoform activated in melanoma cells (19). Thus, we determined whether constitutive activation of Akt3 was able to protect B-RAF targeted WM793 cells against apoptosis in 3-D collagen. Addition of the myristylation signal of the c-Src kinase to the amino terminus of Akt3 strongly enhances Akt3 activation and transforming activity in fibroblast focus forming assays (29). We generated wild-type and myristylated human Akt3 lentiviruses and, subsequently, WM793 cell populations that stably express the Akt3 transgenes. Expression was confirmed by Western blotting with Akt and epitope-tag antibodies (Fig. 3A). Myristylated Akt3 but not wild-type Akt3 led to increased levels of phospho-Akt. Next, we determined the effect of adhesion on Akt signaling in the ectopic Akt-expressing cell populations. While activation of endogenous Akt and wild-type Akt3 was adhesion-dependent, myristylated Akt3 activity was constitutive and adhesion-independent, as indicated by phospho-Akt staining (Fig. 3B). Furthermore, myristylated Akt3 enhanced phosphorylation of GSK-3β, an Akt substrate, and levels of a prominent staining band detected by a phospho-Akt substrate antibody. To determine the effect of constitutive Akt3 signaling on B-RAF targeted cells, we knocked down B-RAF in Akt3-expressing cell lines. By annexin-V staining, expression of myristylated Akt3 partially protected B-RAF knockdown cells from apoptosis in 3-D collagen, whereas wild-type Akt3 was not protective (Fig. 3C). Similarly, expression of myristylated Akt3 but not wild-type Akt3 protected WM793 cells in 3-D collagen gels from PLX4720-induced apoptosis (Fig. 3D). Notably, expression of myristylated Akt3 did not enhance phospho-ERK1/2 in B-RAF knockdown cells (Supplemental Fig. 2).
Figure 3.
Expression of constitutively active Akt3 protects melanoma cells from B-RAF inhibition. A, Whole cell lysates from WM793, WM793 HA-Akt3 or WM793 Myr-HA-Akt3 cells were analyzed by Western blotting for HA, total Akt and phosphoS473-Akt. Actin is the loading control. B, WM793, WM793 HA-Akt3 or WM793 Myr-HA-Akt3 cells were serum starved overnight and replated on agar or fibronectin-coated dishes for 1 h in serum-free medium. Cell lysates were analyzed by Western blotting for phospho-S473 Akt, total Akt, phospho GSK3β, GSK3β, and phospho Akt substrate. Actin was used as a loading control. C, WM793, WM793 HA-Akt3 or WM793 Myr-HA-Akt3 cells were transfected with either control or B-RAF siRNAs for 72 h. Cells were then seeded in 3-D collagen gels and cultured in serum-free medium. After 48 h, cells were analyzed by annexin V staining. D, Serum starved WM793, WM793 HA-Akt3 or WM793 Myr-HA-Akt3 cells were seeded in 3-D collagen gels and cultured in serum-free medium containing DMSO or 0.5 μM PLX4720. After 48 h, cells were analyzed as in C. The quantitation of mean −/+ SD from three independent experiments is shown.
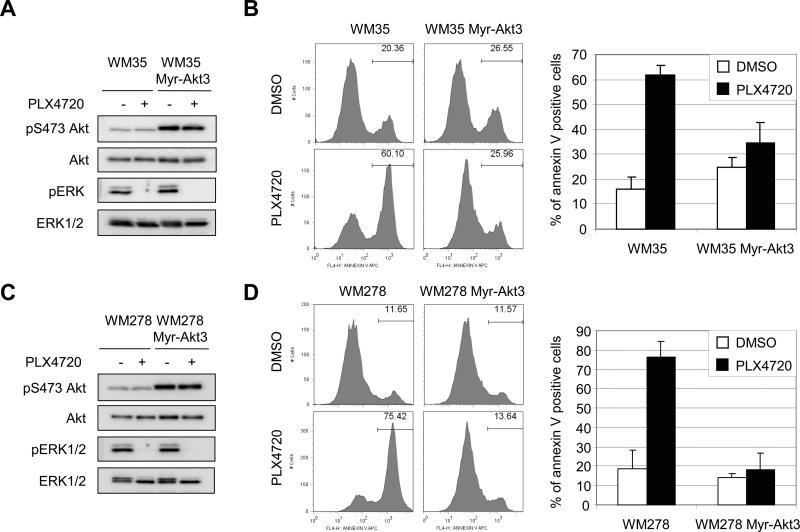
To determine the generality of these effects, we expressed myristylated Akt3 in other mutant B-RAF harboring melanoma cells. WM35 and WM278 cells are B-RAFV600E-expressing and representative of RGP and VGP, respectively. Both Bim-EL and Bmf were up-regulated by PLX4720 in these cells (data not shown). Western blotting confirmed that myristylated Akt3 was highly active and that PLX4720 inhibited ERK1/2 phosphorylation in WM35 cells (Fig. 4A). Importantly, expression of myristylated Akt3 protected WM35 cells from apoptosis induced by PLX4720 in 3-D collagen (Fig. 4B). Similarly, myristylated Akt3-transduced WM278 cells displayed enhanced phosphoAkt and PLX4720 inhibited ERK1/2 in these cells (Fig. 4C). WM278 cells were more sensitive to PLX4720 treatment compared to WM793 cells but myristylated Akt3-WM278 cells still exhibited resistance to PLX4720-induced apoptosis in 3-D collagen (Fig. 4D). Together these data show that constitutive activation of Akt3 protects B-RAF targeted primary stage melanoma cells from apoptosis.
Figure 4.
Myristylated Akt3 protects WM35 and WM278 cells from PLX4720-induced apoptosis in 3-D. A, WM35 and WM35 Myr-HA-Akt3 were treated with DMSO or 1 μM PLX4720 for 1 h. Cell lysates were analyzed by Western blotting for phosphoS473 Akt, total Akt, phospho ERK1/2 and total ERK1/2. B, Serum starved WM35 and WM35 Myr-HA-Akt3 cells were seeded in 3-D collagen gels and cultured in serum-free medium containing DMSO or 1 μM PLX4720. After 48 h, cells were analyzed by annexin V staining. The quantitation of mean −/+ SD from three independent experiments is shown. C, As for A, except, WM278 and WM278 Myr-HA-Akt3 were utilized. D, As for B, except, WM278 and WM278 Myr-HA-Akt3 were utilized and cells were harvested 24 h following PLX4720 treatment.
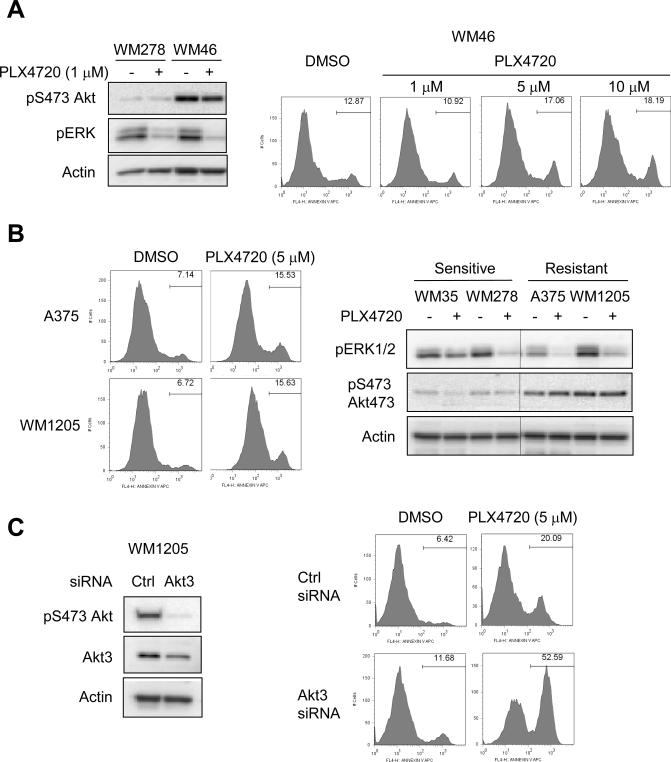
PLX4720 resistant cell lines display elevated Akt phosphorylation in 3-D collagen
A small percentage of melanoma lines harbor Akt3 mutations (30). One identified Akt3 mutant line is WM46, which harbors an E17K mutation that enhances Akt association with the plasma membrane and increases Akt kinase activity (31). WM46 displayed enhanced Akt phosphorylation in 3-D collagen and resistance to PLX4720-induced apoptosis (Fig. 5A). Even treatment with high concentrations of PLX4720 (5–10 μM) were unable to promote substantial apoptosis in WM46 cells. During our studies we identified two metastatic lines, A375 and 1205Lu, that were de novo resistant to PLX4720-induced apoptosis in 3-D collagen (Fig. 5B). Both A375 and 1205Lu displayed enhanced Akt phosphorylation in 3-D compared to the sensitive lines, WM35 and WM278. Furthermore, knockdown of Akt3 rendered 1205Lu cells sensitive to PLX4720-induced apoptosis in 3-D (Fig. 5C). These data further associate elevated Akt phosphorylation with resistance to PLX4720.
Figure 5.
Akt activity in 3-D is elevated in PLX4720-resistant cell lines. A, WM278 and WM46 cells were seeded into 3-D collagen gels −/+ PLX4720 (1, 5 and 10 μM, as indicated). Cells were lysed after 24 hours for Western blot analysis for phosphoS473 Akt, phospho ERK1/2 and actin (left panels). WM46 cells were analyzed after 48 hours for annexin V staining (right panels). B, A375 and 1205Lu cells were seeded into 3-D collagen gels. Left panels show annexin V staining following 48 hours treatment with 5 μM PLX4720. Right panels show Western blot analysis for phosphoS473 Akt, phospho ERK1/2 and actin in A375 and 1205Lu cells in 3-D compared to WM35 and WM278. C, 1205Lu cells were transfected with control non-targeting or Akt3 siRNAs for 72 hours. Cells were lysed for Western blot analysis for phosphoS473 Akt, Akt3 and actin (left panels). Cells were seeded in 3-D collagen gels −/+ 5 uM PLX4720. After 48 hours, cells were harvested for annexinV staining and flow cytometry analysis (right panels).
Myristylated Akt3 prevents up-regulation of Bim-EL and Bmf mRNA following B-RAF inhibition
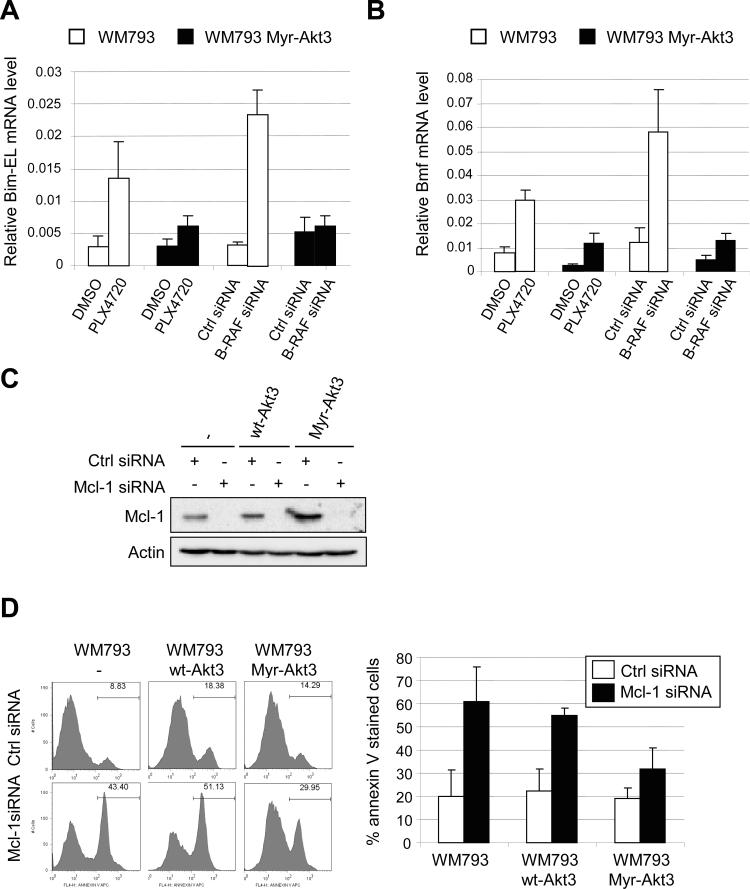
Next, we examined the effect of Akt3 signaling on the induction of Bim-EL and Bmf following B-RAF knockdown and PLX4720 treatment in 3-D collagen. Total RNA was extracted from WM793 cells in collagen gels and analyzed by qRT-PCR. As expected, Bim-EL and Bmf mRNA levels were increased in 3-D following B-RAF knockdown (Fig. 6A and 6B). In cells expressing myristylated Akt3, the up-regulation of both Bim-EL and Bmf was inhibited. Similarly, treatment with PLX4720 in WM793 cells in 3-D led to up-regulation of Bim-EL and Bmf, which was inhibited in cells expressing myristylated Akt3 (Fig. 6A and 6B). Thus, the protective effect of myristylated Akt3 in B-RAF targeted cells is associated with an impaired up-regulation of Bim-EL and Bmf mRNA.
Figure 6.
Myristylated Akt3 counteracts the up-regulation of BH3-only proteins and protects against Mcl-1 knockdown induced apoptosis in 3-D. A, WM793 and WM793 Myr-HA-Akt3 cells were transfected with either control or B-RAF siRNAs for 72 h. Cells were then seeded in 3-D collagen gels and cultured in serum-free medium. After 24 h, cells were harvested for total RNA isolation and qRT-PCR analysis. Additionally, WM793 and WM793 Myr-HA-Akt3 cells were treated with 0.5 μM PLX4720 for 40 h prior to harvesting cells. Quantitation of data from three independent experiments is represented as the mean relative mRNA level of Bim-EL in each condition. B, As for A, except that Bmf mRNA levels were analyzed. C, WM793, WM793 HA-AKT3 or WM793 Myr-HA-AKT3 cells were transfected with control or Mcl-1 siRNA. Seventy-two hours post-transfection cell lysates were harvested and analyzed by Western blotting for Mcl-1 and actin. D, WM793, WM793 HA-AKT3 or WM793 Myr-HA-AKT3 cells transfected with control or Mcl-1 siRNAs were cultured in 3-D collagen gels in serum-free conditions. After 48 h, cells were harvested and stained with annexin V-APC for flow cytometry. The quantitation of mean −/+ SD from four independent experiments is shown.
Activated Akt3 promotes cell survival in Mcl-1 depleted melanoma cells in 3-D
We have previously shown that down-regulation of Mcl-1 renders melanoma cells susceptible to apoptosis in suspension (14). Extending these observations to 3-D, efficient down-regulation of Mcl-1 promoted apoptosis in collagen gels (Fig. 6C and 6D). If Akt3 protective effects were mediated through Bim-EL and Bmf, we would predict that myristylated Akt3 would elicit protective effects in Mcl-1 knockdown cells. Expression of myristylated Akt3 but not wild-type Akt3 was sufficient to protect Mcl-1 knockdown cells from apoptosis (Fig. 6D). Notably, the protection was not as complete as effects elicited by myristylated Akt3 in PLX4720-treated cells; nevertheless, these data suggest that Akt3 signaling regulates apoptotic signaling events, at least in part, through Bim-EL and Bmf.
Discussion
The identification of B-RAF mutations in melanoma, and to a lesser extent in thyroid carcinoma, colorectal cancer and ovarian cancer, has focused efforts on targeted therapeutic strategies to treat these malignancies. Here, we have utilized inhibitor and RNA interference approaches to determine the effect of targeting B-RAF in primary stage melanoma cells cultured in 3-D dermal-like matrices. It is critical to test the importance of tumor cell signaling pathways in ECM microenvironments that more closely resemble the in vivo situation compared to 2-D cultures. Other groups have shown that Rho GTPase activity and focal adhesion kinase signaling differ between 3-D and 2-D conditions (32, 33). Furthermore, the 3-D environment also controls cellular differentiation (34) and has been utilized in breast and prostate cancer progression models (35, 36).
Initially, our study shows that either B-RAF depletion or treatment with the recently described mutant selective B-RAF inhibitor (7), PLX4720, promotes apoptosis in 3-D collagen gels. The effects that we observe are similar to the susceptibility of B-RAF targeted cells to apoptosis in suspension conditions (13). While primary stage cell lines used in this study are sensitive to PLX4720-induced apoptosis, some metastatic lines displayed elevated Akt phosphorylation in 3-D and were less susceptible. Addition of fibronectin to 3-D collagen gels elicited partial protection to PLX4720-induced apoptosis in WM35 cells and low level of protection was observed in two other susceptible cells lines (data not shown). The effects may not be as dramatic as in 2-D due to inefficient remodeling of fibronectin or lower stiffness of the 3-D system.
Enhanced apoptosis in B-RAF targeted cells in 3-D was dependent on the BH3-only proteins, Bim-EL and Bmf. We and others have previously shown that Bim-EL, is up-regulated following B-RAF knockdown or MEK inhibition in melanoma cells and sensitizes melanoma cells to anoikis (13, 23, 24). Up-regulation of Bim-EL occurs through mRNA regulation and protein stability mechanisms (13). Others have suggested that cytosolic accumulation of Bmf correlates with apoptotic sensitivity to MEK inhibition (25); however, the effect of altered Bmf expression in melanoma cells remains unclear. In our studies, Bmf mRNA levels were up-regulated following B-RAF knockdown. These findings are consistent with recent microarray analyses (37). We were unable to verify the specificity of commercial Bmf antibodies, similar to concerns raised by other laboratories (26). While both Bim-EL and Bmf mRNA levels are up-regulated by loss of adhesion in epithelial and fibroblast cells (26, 38, 39), Bim-EL and Bmf mRNA levels were not adhesion regulated in mutant B-RAF melanoma cells (data not shown). Bmf was originally identified in a screen as a binding partner for Mcl-1 (40) and ERK1/2 activity regulates Mcl-1 protein stability in melanoma cells (14). We show that enhanced expression of Mcl-1 provides melanoma cells with resistance to PLX4720-induced apoptosis. Mcl-1 expression in melanoma may underlie resistance to several distinct types of pro-apoptotic signals including resistance to dacarbazine treatment (41), exposure to ionizing radiation (42), the proteasome inhibitor, Bortezomib (43), and endoplasmic reticulum stress (44). These findings suggest that BH3 mimetics that target Mcl-1 may prove therapeutically useful in the treatment of melanoma.
Adhesion of melanoma cells to fibronectin but not type I collagen activates Akt, an effect observed even in PTEN deficient cells such as WM793 (12). Akt3 is the main Akt isoform activated in melanoma (19) and we show that ectopically expressed myristylated Akt3 signals in an adhesion independent manner. Furthermore, constitutive Akt3 activity rendered melanoma cells resistant to B-RAF targeting via either B-RAF knockdown or PLX4720 treatment. Akt3 actions are, at least in part, mediated through preventing the up-regulation of Bim-EL and Bmf induced by B-RAF inhibition. Others have implicated activated Akt in the suppression of Bim-EL and Bmf in non-melanoma cell types (26, 45) and these findings indicate that other Akt isoforms may elicit similar effects. We have not yet identified the downstream effectors of Akt3 that mediate effects on Bim-EL and Bmf; however, based on others findings mTOR, PRAS40 and GSK-3β are likely mediators (46–48). Similar to B-RAF knockdown/inhibition, depletion of the pro-survival protein Mcl-1 renders melanoma cells susceptible to apoptosis in 3-D. Mcl-1 knockdown induced apoptosis was protected by expression of myristylated Akt3; however, the effect was partial suggesting that additional pathways may be involved.
In summary, we show that constitutive, adhesion-independent Akt3 activity confers resistance to melanoma cell apoptosis induced by B-RAF targeting in 3-D. Our findings underscore the importance of combinatorial targeting of both the B-RAF-MEK and integrin-PI-3 kinase-Akt signaling pathways to elicit cytotoxic effects. Dual targeting of these two pathways inhibits melanoma tumors in xenografts and causes tumor regression of B-RAFV600E/loss of PTEN-induced melanomas in mouse models (20, 46, 49). Furthermore, overexpression of activated Akt1 enhances tumor forming ability of the non-tumorigenic cell line, WM35 (50). A small percentage of melanoma cells harbor Akt3 mutations (30); nevertheless, mutations in Akt3 or other components of this pathway represent possible mechanisms of de novo and/or acquired resistance to B-RAF inhibitors.
Supplementary Material
Acknowledgements
Grant support: National Institutes of Health grants, R01-GM067893 and R01-CA125103, and American Cancer Society grant, RSG-08-03-01-CSM, to A.E. Aplin.
We are grateful to Dr. Gideon Bollag (Plexxikon) for providing PLX4720, Dr. Meenhard Herlyn (Wistar Institute) for cell lines, Dr. Steven Edwards (Liverpool, UK) for providing the Mcl-1 cDNA, and the Flow Cytometry shared resource in the Kimmel Cancer Center.
Abbreviations
- Bim-EL
Bcl-2 interacting mediator extra long
- Bmf
Bcl-2-modifying factor
- ECM
extracellular matrix
- Mcl-1
myeloid cell leukemia-1
- RGP
radial growth phase
- VGP
vertical growth phase
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed
References
- 1.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Cohen C, Zavala-Pompa A, Sequeira JH, et al. Mitogen-actived protein kinase activation is an early event in melanoma progression. Clin Cancer Res. 2002;8:3728–33. [PubMed] [Google Scholar]
- 3.Satyamoorthy K, Li G, Gerrero MR, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–9. [PubMed] [Google Scholar]
- 4.Hingorani SR, Jacobetz MA, Robertson GP, Herlyn M, Tuveson DA. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res. 2003;63:5198–202. [PubMed] [Google Scholar]
- 5.Karasarides M, Chiloeches A, Hayward R, et al. B-RAF is a therapeutic target in melanoma. Oncogene. 2004;23:6292–8. doi: 10.1038/sj.onc.1207785. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt KV, Spofford LS, Aram G, McMullen M, Pumiglia K, Aplin AE. Adhesion control of cyclin D1 and p27Kip1 levels is deregulated in melanoma cells through BRAF-MEK-ERK signaling. Oncogene. 2005;12:3459–71. doi: 10.1038/sj.onc.1208544. [DOI] [PubMed] [Google Scholar]
- 7.Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA. 2008;105:3041–6. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sondak VK, Smalley K. Targeting mutant BRAF and KIT in metastatic melanoma: ASCO 2009 meeting report. Pigment Cell Melanoma Res. 2009;22:386–7. doi: 10.1111/j.1755-148X.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- 9.Wellbrock C, Ogilvie L, Hedley D, et al. V599EB-RAF is an oncogene in melanocytes. Cancer Res. 2004;64:2338–42. doi: 10.1158/0008-5472.can-03-3433. [DOI] [PubMed] [Google Scholar]
- 10.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott G, Ryan DH, McCarthy JB. Molecular mechanisms of human melanocyte attachment to fibronectin. J Invest Dermatol. 1992;99:787–94. doi: 10.1111/1523-1747.ep12614749. [DOI] [PubMed] [Google Scholar]
- 12.Boisvert-Adamo K, Aplin AE. B-RAF and PI-3 kinase signaling protect melanoma cells from anoikis. Oncogene. 2006;25:4848–56. doi: 10.1038/sj.onc.1209493. [DOI] [PubMed] [Google Scholar]
- 13.Boisvert-Adamo K, Aplin AE. Mutant B-RAF mediates resistance to anoikis via Bad and Bim. Oncogene. 2008;27:3301–12. doi: 10.1038/sj.onc.1211003. [DOI] [PubMed] [Google Scholar]
- 14.Boisvert-Adamo K, Longmate W, Abel EV, Aplin AE. Mcl-1 is required for melanoma resistance to anoikis. Mol Cancer Res. 2009;7:549–56. doi: 10.1158/1541-7786.MCR-08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albelda SM, Mette SA, Elder DE, et al. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990;50:6757–64. [PubMed] [Google Scholar]
- 18.Humphries MJ, Olden K, Yamada KM. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986;233:467–70. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
- 19.Stahl JM, Sharma A, Cheung M, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–10. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 20.Cheung M, Sharma A, Madhunapantula SV, Robertson GP. Akt3 and mutant V600EB-Raf cooperate to promote early melanoma development. Cancer Res. 2008;68:3429–39. doi: 10.1158/0008-5472.CAN-07-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akgul C, Moulding DA, White MRH, Edwards SW. In vivo localisation and stability of human Mcl-1 using green fluorescent protein (GFP) fusion proteins. FEBS Lett. 2000;478:72–6. doi: 10.1016/s0014-5793(00)01809-3. [DOI] [PubMed] [Google Scholar]
- 22.Henriet P, Zhong ZD, Brooks PC, Weinberg KI, DeClerck YA. Contact with fibrillar collagen inhibits melanoma cell proliferation by up-regulating p27KIP1. Proc Natl Acad Sci USA. 2000;97:10026–31. doi: 10.1073/pnas.170290997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verhaegen M, Bauer JA, Martin de la Vega C, et al. A novel BH3 mimetic reveals a mitogen-activated protein kinase-dependent mechanism of melanoma cell death controlled by p53 and reactive oxygen species. Cancer Res. 2006;66:11348–59. doi: 10.1158/0008-5472.CAN-06-1748. [DOI] [PubMed] [Google Scholar]
- 24.Cartlidge RA, Thomas GR, Cagnol S, et al. Oncogenic BRAF(V600E) inhibits BIM expression to promote melanoma cell survival. Pigment Cell Melanoma Res. 2008;21:534–44. doi: 10.1111/j.1755-148X.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanBrocklin MW, Verhaegen M, Soengas MS, Holmen SL. Mitogen-activated protein kinase inhibition induces translocation of Bmf to promote apoptosis in melanoma. Cancer Res. 2009;69:1985–94. doi: 10.1158/0008-5472.CAN-08-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmelzle T, Mailleux AA, Overholtzer M, et al. Functional role and oncogene-regulated expression of the BH3-only factor Bmf in mammary epithelial anoikis and morphogenesis. Proc Natl Acad Sci USA. 2007;104:3787–92. doi: 10.1073/pnas.0700115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 28.Merino D, Giam M, Hughes PD, et al. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol. 2009;186:355–62. doi: 10.1083/jcb.200905153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mende I, Malstrom S, Tsichlis P, Vogt P, Aoki M. Oncogenic transformation induced by membrane-targeted Akt2 and Akt3. Oncogene. 2001;20:4419–23. doi: 10.1038/sj.onc.1204486. [DOI] [PubMed] [Google Scholar]
- 30.Davies MA, Stemke-Hale K, Tellez C, et al. A novel AKT3 mutation in melanoma tumours and cell lines. Br J Cancer. 2008;99:1265–8. doi: 10.1038/sj.bjc.6604637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–44. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 32.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 33.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583–95. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci. 2003;116:2377–88. doi: 10.1242/jcs.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen O, Ronnov-Jessen L, Howlett A, Bissell M. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–8. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lang SH, Sharrard RM, Stark M, Villette JM, Maitland NJ. Prostate epithelial cell lines form spheroids with evidence of glandular differentiation in three-dimensional Matrigel cultures. Br J Cancer. 2001;85:590–9. doi: 10.1054/bjoc.2001.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Packer LM, East P, Reis-Filho JS, Marais R. Identification of direct transcriptional targets of V600E BRAF/MEK signalling in melanoma. Pigment Cell Melanoma Res. 2009;22:785–98. doi: 10.1111/j.1755-148X.2009.00618.x. [DOI] [PubMed] [Google Scholar]
- 38.Reginato MJ, Mills KR, Paulus JK, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;6:6. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- 39.Woods NT, Yamaguchi H, Lee FY, Bhalla KN, Wang H-G. Anoikis, initiated by Mcl-1 degradation and Bim induction, is deregulated during oncogenesis. Cancer Res. 2007;67:10744–52. doi: 10.1158/0008-5472.CAN-07-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puthalakath H, Villunger A, O'Reilly LA, et al. Bmf: A proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 2001;293:1829–32. doi: 10.1126/science.1062257. [DOI] [PubMed] [Google Scholar]
- 41.Thallinger C, Wolschek MF, Wacheck V, et al. Mcl-1 antisense therapy chemosensitizes human melanoma in a SCID mouse xenotransplantation model. J Invest Dermatol. 2003;120:1081–6. doi: 10.1046/j.1523-1747.2003.12252.x. [DOI] [PubMed] [Google Scholar]
- 42.Skvara H, Thallinger C, Wacheck V, et al. Mcl-1 blocks radiation-induced apoptosis and inhibits clonogenic cell death. Anticancer Res. 2005;25:2697–703. [PubMed] [Google Scholar]
- 43.Qin JZ, Xin H, Sitailo LA, Denning MF, Nickoloff BJ. Enhanced killing of melanoma cells by simultaneously targeting Mcl-1 and NOXA. Cancer Res. 2006;66:9636–45. doi: 10.1158/0008-5472.CAN-06-0747. [DOI] [PubMed] [Google Scholar]
- 44.Jiang CC, Lucas K, Avery-Kiejda KA, et al. Up-regulation of Mcl-1 is critical for survival of human melanoma cells upon endoplasmic reticulum stress. Cancer Res. 2008;68:6708–17. doi: 10.1158/0008-5472.CAN-08-0349. [DOI] [PubMed] [Google Scholar]
- 45.Stahl M, Dijkers PF, Kops GJPL, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–31. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 46.Dankort D, Curley DP, Cartlidge RA, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–52. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madhunapantula SV, Sharma A, Robertson GP. PRAS40 deregulates apoptosis in malignant melanoma. Cancer Res. 2007;67:3626–36. doi: 10.1158/0008-5472.CAN-06-4234. [DOI] [PubMed] [Google Scholar]
- 48.Panka DJ, Cho DC, Atkins MB, Mier JW. GSK-3 inhibition enhances Sorafenib-induced apoptosis in melanoma cell lines. J Biol Chem. 2008;283:726–32. doi: 10.1074/jbc.M705343200. [DOI] [PubMed] [Google Scholar]
- 49.Tran MA, Gowda R, Sharma A, et al. Targeting V600EB-Raf and Akt3 using nanoliposomal-small interfering RNA inhibits cutaneous melanocytic lesion development. Cancer Res. 2008;68:7638–49. doi: 10.1158/0008-5472.CAN-07-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Govindarajan B, Sligh JE, Vincent BJ, et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007;117:719–29. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.