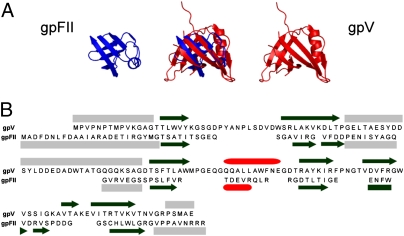
Fig. 2.
Structural comparison of gpFII and gpV. (A) The structural overlay of gpFII and gpV is displayed in the center and the individual monomers are displayed on either side. These structures overlay with an rmsd of 2.4 Å over 46 residues. (B) The sequences of gpFII and gpV are aligned according to the structural alignment. The secondary structure of gpV is indicated above its sequence and that of gpFII is shown below its sequence (β-strands are represented as black arrows, α-helices as red cylinders, and unstructured regions as gray boxes). Unstructured regions of gpFII (Fig. S2A) and gpV (3) were delineated by measuring {1H}-15N heteronuclear NOEs.