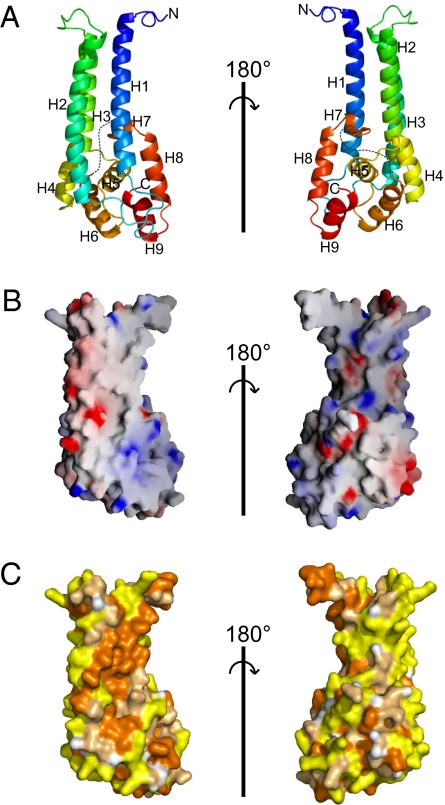
Fig. 1.
Structure of the C-terminal fragment of Vps53 (residues 564–799). (A) Ribbon diagrams colored from blue at the N-terminal end to red at the C terminus. Sequences that are disordered in the structure are indicated by dotted lines. Two orientations, related by a 180° rotation, are shown. (B) The surface of the Vps53 C terminus (oriented as in the ribbon diagrams) is colored according to electrostatic potential. (C) The surface is colored according to hydrophobicity. Hydrophobic residues (Val, Ile, Leu, Pro, Met, Ala, Phe, and Trp) are orange, polar residues (Ser, Thr, Cys, Asn, Gln, and Tyr) are pale orange, and charged residues (Glu, Asp, Lys, Arg, and His) are yellow.