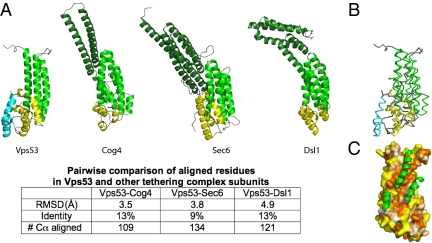
Fig. 2.
The C-terminal fragment has a similar fold to subunits in the COG, exocyst, and Dsl1 multisubunit tethering complexes. (A) Vps53, Cog4 (PDB ID 3HR0), Sec6 (PDB ID 2FJI), and Dsl1 (PDB ID 3K8P) C-terminal fragments are shown in the same orientation. Distinct helical bundles are shown in dark green, green, and yellow (at the C terminus). The C-terminal helical bundle in Vps53 has two helices not present in the other proteins; they are shown in cyan. The N-terminal-most helical bundle, or “stem,” in the Vps53 fragment lacks two or three helices present in the other proteins. A more quantitative comparison of the structures is tabulated below. (B) Superposition of the Vps53 and Cog4 C termini, showing that Cog4 lacks the two most C-terminal helices in Vps53 (cyan). Vps53 and Cog4 are colored as in A. (C) The surface of the C-terminal fragment of Vps53, with residues colored according to hydrophobicity as in Fig. 1C. The additional helices present in the Cog4 “stem” are indicated in green. They bury the Vps53 hydrophobic patch, suggesting that the full-length Vps53 protein shares these helices even as they are not present in the proteolytic fragment of Vps53 that was crystallized.