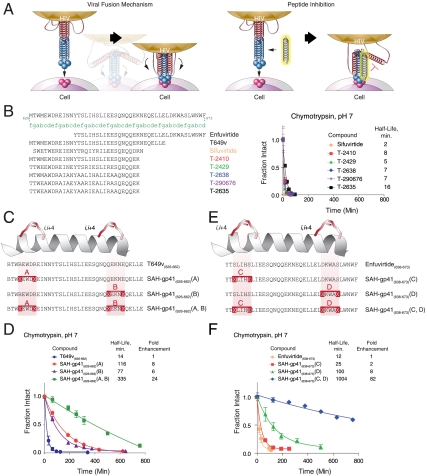
Fig. 1.
Design and synthesis of SAH-gp41 peptides. (A) Schematic of the HIV-1 viral fusion mechanism and its disruption by a decoy HR2 helix. (B) A series of reported next-generation gp41 HR2 peptides (18, 20), which contain natural amino acid substitutions, helix-promoting alanine residues, and/or (i, i + 4) salt bridges, exhibited rapid proteolytic degradation upon exposure to chymotrypsin. (C) SAH-gp41 peptides were designed based on gp41 HR2 domain sequences 626–662 (a variant of T649). X, substitution sites for crosslinking nonnatural amino acids; B, norleucine (substituted for methionine to optimize activity of the ruthenium catalyst). (D) Upon exposure to chymotrypsin, the singly stapled SAH-gp41 peptides exhibited 6–8-fold longer half-lives compared to the unmodified peptide, whereas double-stapling conferred a 24-fold enhancement in protease resistance. (E) Singly and doubly stapled SAH-gp41 peptides were also constructed based on the enfuvirtide peptide sequence (638–673). (F) Like SAH-gp41(626–662) peptides, the singly stapled SAH-gp41(638–673) derivatives showed enhanced chymotrypsin resistance compared to the template peptide, and the doubly stapled peptide was strikingly more resistant to proteolysis. Fraction intact, mean ± s.d.