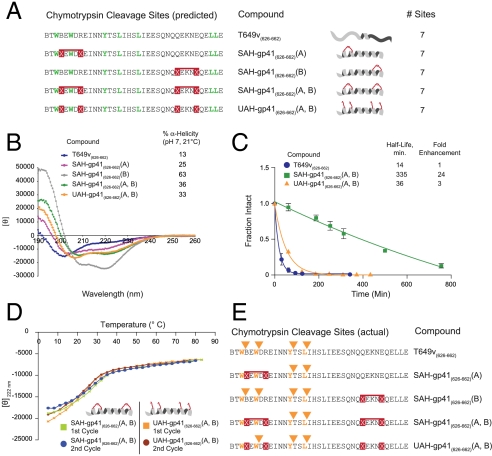
Fig. 2.
Mechanistic analysis of peptide fortification by hydrocarbon double-stapling. (A) T649v and its synthetic derivatives contain the identical number of chymotrypsin cleavage sites. (B) CD demonstrated that T649v is predominantly a random coil in solution, whereas SAH-gp41(626–662) peptides exhibited varying degrees of increased α-helical content. UAH- and SAH-gp41(626–662) (A, B) displayed similar α-helical content, which was intermediate to that of the singly stapled peptides. (C) UAH-gp41(626–662)(A, B) exhibited an 8-fold shorter half-life than its doubly stapled counterpart. Fraction intact, mean ± s.d. (D) UAH- and SAH-gp41(626–662)(A, B) displayed similar melting profiles, with Tm values of 27 and 22 °C, respectively. Temperature-dependent unfolding was reversible for both peptides, as evidenced by the overlapping repeat melting curves. (E) Comparative chymotrypsin degradation patterns of T649v-based peptides. Of note, the N-terminal staple uniquely prevented proteolytic hydrolysis of the cleavage site flanked by the staple, with no corresponding M + 18 species observed by LC/MS.