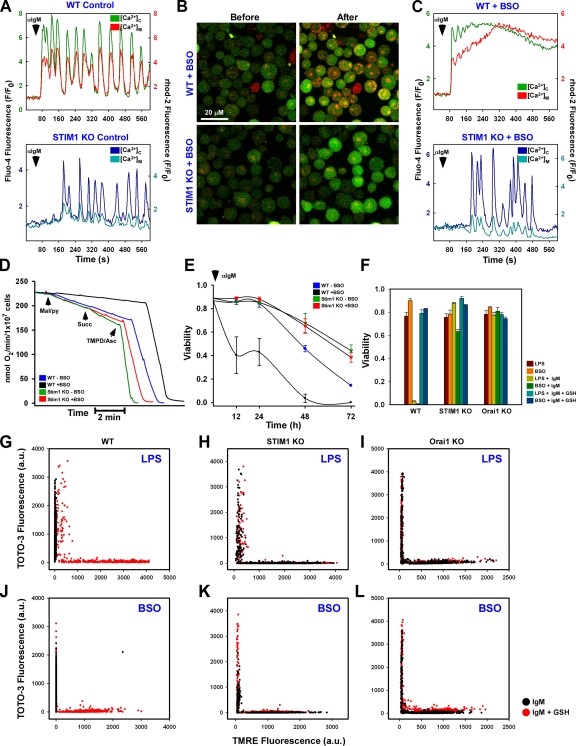
Figure 4.
The CRAC channel is requisite for oxidative stress-induced alterations in mitochondrial Ca2+ handling, bioenergetics, and cell death. Fluo-4– and rhod-2–loaded DT40 cells were stimulated with αIgM and fluorescence changes recorded via confocal microscopy. (A) Coordinated cytosolic and mitochondrial Ca2+ levels in control DT40 cells. (B) Confocal images before and after αIgM addition in BSO-pretreated WT (top) and STIM1 KO (bottom) cells. (C) Representative single-cell traces of cytosolic and mitochondrial Ca2+ levels after αIgM stimulation in BSO-treated DT40 cells. (D) Oxygen consumption in DT40 cells in response to complex I (malate/pyruvate), complex II/III (succinate), and complex IV (TMPD/ascorbate) substrates. Traces are representative of at least five independent experiments. (E) Sensitization of DT40 cells to 1.5 µg/ml αIgM-induced cell death by BSO, as determined by nuclear incorporation of TOTO-3. αIgM was added to cells every 24 h. Values were determined by counting TOTO-3–positive cells in five independent fields at the indicated time points via confocal microscopy (n = 3). (F) DT40 cells were sensitized with either 1 µg/ml LPS for 5 h or 200 µM BSO for 24 h and treated with 1.5 µg/ml αIgM for 48 h either in the absence or presence of 2.5 mM GSH. Cell viability was assessed as the percentage of TOTO-3–negative cells in five independent fields (n = 3). DT40 cells were simultaneously loaded with TOTO-3 and TMRE to assess plasma membrane integrity and Δψm, respectively. (G and J) Functional mitochondria were not observed in either LPS (G) or BSO-sensitized WT DT40 (J) cells, but TMRE fluorescence could be restored by supplementation with 2.5 mM GSH. (H, I, K, and L) LPS and BSO had a trivial effect on either STIM1 KO (H and K) or Orai1 KO (I and L) cells. Error bars indicate mean ± SEM.