Figure 1.
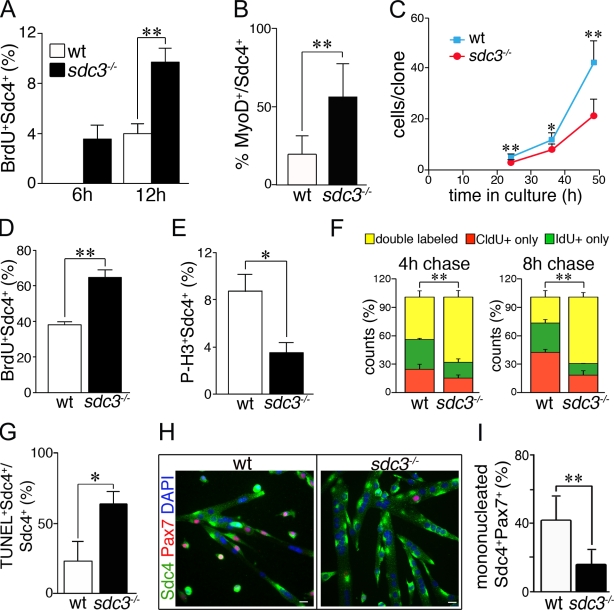
Cell cycle progression and cell fate decisions are dysregulated in cultured sdc3−/− SCs. (A and B) Myofiber-associated sdc3−/− SCs exit from quiescence and enter the cell cycle faster than wild-type cells. The percentage of BrdU+ (A) and MyoD+ (B) SCs is increased on sdc3−/− myofibers at 6 h and 12 h after explant compared with wild-type myofibers. (C) Sdc3−/− SCs expand less than wild-type controls during the first 2.5 d in culture (means from one representative experiment of four; ≥20 clones/plate scored). (D and E) Explanted and cultured sdc3−/− SCs were pulse-labeled for 30 min with 10 µM BrdU before fixation and immunostaining to detect BrdU, Syndecan-4 (Sdc4) as a SC marker, and P-H3 to mark mitotic cells. The percentage of Sdc4+BrdU+ cells normalized to the total number of Sdc4+ cells is higher (D, n = 5) and the percentage of Sdc4+P-H3+ cells is lower (E, n = 3) in sdc3−/− SC cultures compared with wild-type controls. (F) Wild-type and sdc3−/− SCs in culture were pulse-labeled with CldU followed by IdU after either a 4-h or 8-h chase. A higher percentage of sdc3−/− cells were in S phase during both the CldU and the IdU pulses, and were therefore double labeled, compared with wild-type cells. (G) Increased cell death is detected by a TUNEL assay in explanted sdc3−/− SC cultures compared with wild-type cultures (n = 3). (H) Explanted sdc3−/− SCs fuse into hypertrophic myotubes and generate fewer Sdc4+/Pax7+ reserve cells compared with wild-type cultures 48 h after differentiation induction. Bars, 10 µm. (I) Quantification of G. Pax7+Sdc4+ mononuclear cells normalized to total Sdc4+ mononuclear cells (15 clones/sample; n = 3). Open bars, wild type; shaded bars, sdc3−/−. Error bars indicate SEM. **, P < 0.01; *, P < 0.05.