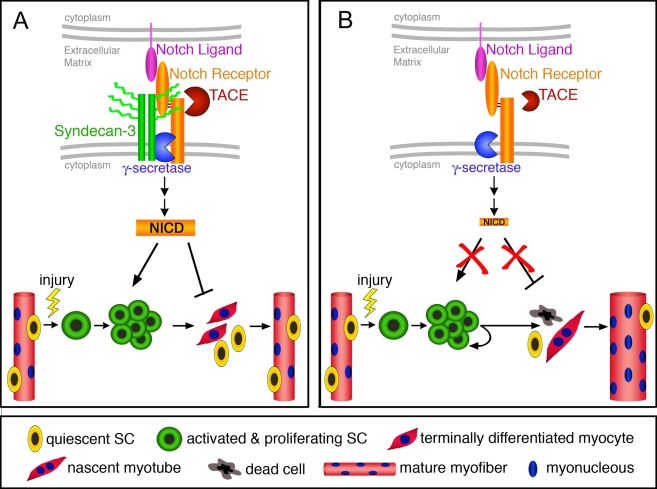
Figure 7.
A model predicting SC behavior in sdc3−/− muscles. (A) In wild-type SCs, Syndecan-3 and Notch reside in a complex where Syndecan-3 facilitates Notch processing and NICD generation. Quiescent SCs (yellow cells) are activated and commit to myogenesis (green cells), where cooperation between Syndecan-3 and Notch promotes cell cycle progression and self-renewal, and inhibits terminal differentiation (red cells) and fusion to existing myofibers. Once muscle repair is completed, SCs cease proliferation and undifferentiated cells return to a quiescent state. Repaired myofibers appear indistinguishable from uninjured myofibers. (B) In sdc3−/− SCs, Notch processing is impaired and NICD generation is dramatically reduced. Once sdc3−/− SCs are activated and commit to myogenesis, the lack of Syndecan-3 and Notch signaling impairs cell cycle progression, delays onset of terminal differentiation, and reduces SC self-renewal. Activated sdc3−/− SCs fail to return to quiescence but remain in an activated state that leads to myofiber hypertrophy. An increase in cell death and the lengthening of cell cycle progression contribute in maintaining constant the total number of nuclei per tissue area. However, the balance between terminal differentiation and self-renewal is disrupted in sdc3−/− regenerating muscles, whereby more nuclei are found in hypertrophic myofibers and less in mononucleated sublaminar Pax7+ cells (SCs). Notch-independent Syndecan-3-dependent signaling pathways are not depicted in this diagram.