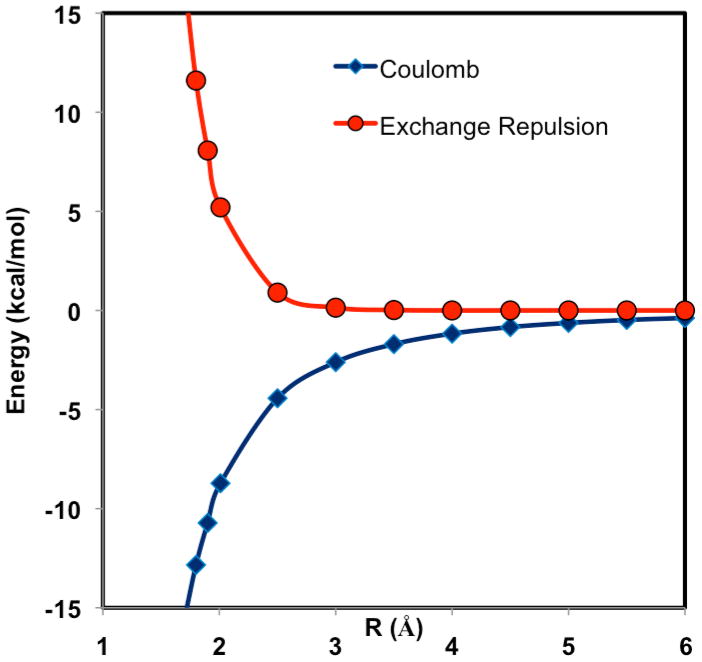
Figure 3.
Computed Coulomb (X-Pol) and exchange repulsion (X-Pol-X) interaction energy as a function of the hydrogen bond distance between the donor hydrogen and the acceptor oxygen atoms in the water dimer complex with Cs symmetry. Energies are computed using the 6-31+G(d) basis at the fully optimized geometries under the Cs symmetry constraint at the HF/6-31+G(d) level. The interaction energy profile is obtained from full geometry optimized with the hydrogen-bond distance specified above held fixed at various values shown in the figure.