Abstract
The zebrafish (Danio rerio) is emerging as a promising model organism for experimental studies of stress and anxiety. Here we further validate zebrafish models of stress by analyzing how environmental and pharmacological manipulations affect their behavioral and physiological phenotypes. Experimental manipulations included exposure to alarm pheromone, chronic exposure to fluoxetine, acute exposure to caffeine, as well as acute and chronic exposure to ethanol. Acute (but not chronic) alarm pheromone and acute caffeine produced robust anxiogenic effects, including reduced exploration, increased erratic movements and freezing behavior in zebrafish tested in the novel tank diving test. In contrast, ethanol and fluoxetine had robust anxiolytic effects, including increased exploration and reduced erratic movements. The behavior of several zebrafish strains was also quantified to ascertain differences in their behavioral profiles, revealing high-anxiety (leopard, albino) and low-anxiety (wild type) strains. We also used LocoScan (CleverSys Inc.) video-tracking tool to quantify anxiety-related behaviors in zebrafish, and dissect anxiety-related phenotypes from locomotor activity. Finally, we developed a simple and effective method of measuring zebrafish physiological stress responses (based on a human salivary cortisol assay), and showed that alterations in whole-body cortisol levels in zebrafish parallel behavioral indices of anxiety. Collectively, our results confirm zebrafish as a valid, reliable, and high-throughput model of stress and affective disorders.
Keywords: Zebrafish, Anxiety, Stress, Novel tank test, Cortisol
1. Introduction
Zebrafish (Danio rerio) have long been utilized as an animal model for biomedical research, particularly in developmental and genetic studies [1]. As a relatively simple vertebrate species, the zebrafish is physiologically homologous to humans, permitting researchers to probe the pathways and mechanisms relevant to human pathogenesis and clinical treatments [2]. Zebrafish possess all of the ‘classical’ vertebrate neurotransmitters [3,4] and their neuroendocrine system provides robust physiological responses to stress [5]. Moreover, zebrafish are an ideal animal model for laboratory research because they are inexpensive, low-maintenance, and abundantly produce offspring [6].
The potential of the zebrafish as a model in neurobehavioral research has emerged only recently. Several studies have examined behavior in zebrafish larvae [7–10], and their responses to different drugs, such as ethanol [11,12] and fluoxetine [13]. In addition to larvae studies, behavioral phenotyping of adult zebrafish also represents an important direction of research [14–17]. Published studies on adult zebrafish include social behavior [18–20], olfactory-related behaviors [21,22], anxiety [23], addiction [24–26], sleep [27], learning and memory [28,29].
As in many other species, exposure to novelty evokes robust anxiety responses in zebrafish [30]. Conceptually similar to the rodent open field test, the novel tank diving test (Fig. 1) exploits the instinctual behavior of zebrafish to seek protection in an unfamiliar environment by diving and remaining at the bottom until they feel safe enough to explore [31]. Using this model, researchers are able to collect and compare behavioral parameters (such as latency to enter the upper half, transitions into the upper half of the tank, erratic movements and freezing bouts) to assess anxiety [23]. Usually, a longer latency to enter the upper half, reduced time spent in the top, as well as increased erratic movements and freezing indicate heightened anxiety [23,31].
Fig. 1.
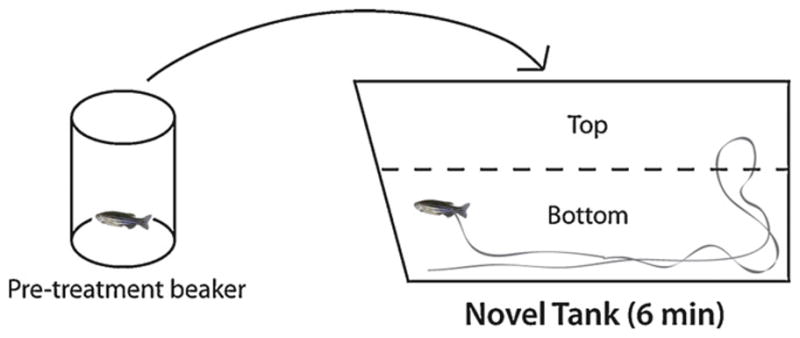
Novel tank diving test. Zebrafish are exposed to the experimental challenge in a pre-treatment beaker before being transferred into the novel tank for behavioral observation and phenotyping. Control groups undergo same procedures without challenge in pre-treatment beaker.
Another method to evoke anxiety in zebrafish is to expose them to alarm pheromone. Originally described in 1941 [32], the pheromone is naturally synthesized in epidermal cells of fish and is released if the cell membrane is damaged (i.e., during predator attack), inducing a strong fear response in nearby conspecifics [33]. In zebrafish, this response is characterized by increased shoal cohesion, faster swimming with spontaneous rapid turns, increased frequency and length of freezing bouts, decreased aggression, and markedly increased bottom dwelling [34].
Finally, anxiety-like behavior can be modulated using various anxiolytic or anxiogenic drugs. Several studies have already examined anxiety in zebrafish using anxiolytic agents such as nicotine [31], α-fluromethylhistidine [35], ethanol [6] and diazepam [36]. Drug-evoked anxiety has also been reported in zebrafish exposed to the benzodiazepine inverse agonist FG-7142 or following abrupt cessation of chronic cocaine administration [36].
When studying anxiety, physiological endpoints, such as stress hormone levels, can also be valuable additions to parallel with behavioral observations [37]. In zebrafish, the hypothalamic–pituitary–interrenal axis is fundamental to stress responses, and involves a cascade of hormones from corticotropin releasing hormone (CRH) to adrenocorticotropic hormone (ACTH) and cortisol. Notably, zebrafish, like humans, employ cortisol (rather than corticosterone, as do rodents) as a primary stress response hormone [23], which makes them a useful animal model relevant to human stress physiology. Lastly, as several strains of zebrafish are currently available for research (Zebrafish International Resource Center, http://zebrafish.org), genetic variability affecting baseline stress responses are also important to consider.
Here we assess zebrafish as a model of stress and anxiety by examining how behavioral and physiological phenotypes are affected by various environmental, pharmacological and genetic factors. Specifically, we wanted to examine zebrafish behavioral responses to a wide spectrum of anxiolytic and anxiogenic factors, and examine whether endocrine stress responses in zebrafish may parallel behavioral phenotypes. This study also aimed to assess whether adult zebrafish anxiety can be reliably quantified using manual and automated registration of their behavior, and whether anxiety and activity phenotypes in zebrafish can be reliably dissected in novelty-based behavioral paradigms. Potentially anxiogenic experimental manipulations used here included acute and prolonged exposure to alarm pheromone and acute exposure to caffeine. Potentially anxiolytic manipulations included chronic exposure to the selective serotonin reuptake inhibitor (SSRI) fluoxetine, as well as acute and chronic ethanol. We also compared several zebrafish strains in order to ascertain baseline behavioral differences in their anxiety phenotypes.
2. Methods
2.1. Animals and housing
Total 336 adult (3–5 month-old) male and female zebrafish were obtained from local commercial distributers (Petco, Rockville, MD and 50 Fathoms, Metarie, LA). Our study included one “wild type” (short fin) zebrafish strain (n = 293) and three mutant strains: long-fin (n = 15), leopard (n = 13) and albino (n = 15). All fish were given at least 10 days to acclimate to the laboratory environment and housed in groups of 20–30 fish per 40-L tank. All tanks were filled with deionized water treated with Prime Freshwater and Saltwater Concentrated Conditioner (Seachem Laboratories, Inc., Madison, GA). The room and water temperatures were maintained at 25–27 °C. Illumination was provided by ceiling-mounted fluorescent light tubes on a 12-h cycle (on: 8.00, off: 20.00). Fish were fed Tetramin Tropical Flakes (Tetra USA, Blacksburg, VA). All fish used in this study were experimentally naïve. Following behavioral testing, the animals were euthanized in 500 mg/L Tricane (Sigma–Aldrich, USA), and immediately dissected for further analysis.
2.2. Novel tank diving test
After pre-treatment, zebrafish were placed individually in a 1.5-L trapezoidal tank (15.2 height × 27.9 top × 22.5 bottom × 7.1 width cm; Fig. 1; Aquatic Habitats, Apopka, FL) maximally filled with aquarium treated water. Novel tanks rested on a level, stable surface and were divided into two equal virtual horizontal portions, marked by a dividing line on the outside walls. Once relocated to novel tanks, zebrafish swimming behavior was recorded by two trained observers (inter-rater reliability >0.85) over a 6-min period. The following endpoints were recorded: latency to reach the upper portion of the tank (s), time spent in the upper portion of the tank (s), number of transitions (entries) to the upper portion of the tank, number of erratic movements, number of freezing bouts and freezing duration (s). Erratic movements were defined as sharp changes in direction or velocity and repeated rapid darting behaviors. Freezing was defined as a total absence of movement, except for the gills and eyes, for 1 s or longer. A significant decrease in exploration (longer latency to reach the top, fewer entries to the top, longer freezing) or elevated erratic movements represent behavioral profiles indicative of high stress and anxiety. These endpoints were chosen based on previous zebrafish studies using the novel tank diving paradigm [23,31].
2.3. Alarm pheromone-evoked anxiety
Alarm pheromone was extracted from epidermal cells of euthanized zebrafish. To damage the epidermal cells, 10–15 shallow slices were made on one side of the fish body with a razor blade [33]. Cuts were carefully controlled to prevent drawing blood, which would contaminate the pheromone solution. The body was then washed for 5 min (damaged side down) in a Petri dish filled with 10 mL of distilled water and placed on ice to preserve the extracted pheromone. The dish was shaken gently to fully cover lacerated portions of the animals. The entire procedure was then repeated on the opposite side of the zebrafish. As multiple zebrafish underwent the procedure, extracted alarm pheromone was collected in 50-mL test tubes for experimental use. The alarm pheromone was administered acutely by adding 7 mL of the collected solution to fresh water in the novel tank directly prior to testing. Prolonged exposure was performed in a pre-treatment beaker for 30 min, after which fish were transferred to the novel tank for anxiety testing. Twenty-two fish were used in acute alarm pheromone experiment (n = 11 in each group), and 20 fish (n = 10 in each group) were used in prolonged alarm pheromone study.
2.4. Pharmacological manipulations
For chronic ethanol exposure experiments, home tank water was treated with ethanol (0.3%, v/v, Pharmco-AAPER, USA) for 7 days. Tank water was replaced every 2 days, and ethanol was administered before introducing subjects back into the home tank. Forty-four fish (n = 20–24 in each group) were used in this study. Ethanol was also administered acutely by placing individual zebrafish into 500 mL of 0.3% ethanol for 5 min. Ten acutely exposed fish and 10 control fish were immediately tested in the novel tank diving test.
To assess behavioral and physiological effects of chronic antidepressant administration, the SSRI fluoxetine (100 μg/L, Sigma–Aldrich, USA) was administered daily for 2 weeks to experimental zebrafish (n = 12). Their respective controls (n = 14) did not receive drug treatment during this time, but were housed in otherwise identical conditions. To assess behavioral effects of acute psychostimulant exposure, caffeine (100 mg/L, Sigma–Aldrich, USA) was administered in a 3-L pre-treatment beaker for 5 min, controls underwent the same procedure without caffeine (n = 21 in each group). In all experiments, novel tank diving test was performed in a novel tank containing standard fish-grade water.
2.5. Automated video-tracking of zebrafish behavior
In a separate study, using zebrafish treated chronically with 0.3% ethanol (n = 20–24) or 50 μg/L fluoxetine (n = 16) for 2 weeks, we performed automated registration of behavior. Trials were recorded to a computer using a USB webcam (2.0-Megapixel, Gigaware, UK) for the 6-min observation period and subsequently analyzed using LocoScan (CleverSys Inc., Reston, VA). Within the LocoScan module ‘top’ and ‘bottom’ zones were established and event rules were set to precisely and consistently register behavioral profiles. Subsequently, additional endpoints, such as distance travelled (m) and velocity (m/s), could be gathered for statistical comparison.
2.6. Cortisol assay
The cortisol extraction procedure was modified from Alderman and Bernier [38]. Individual body samples obtained from the fluoxetine-treated behavioral cohort were homogenized in 750 μL of ice-cold 1× PBS buffer. The homogenizing rotor blade was then washed with an additional 250 μL of ice-cold 1× PBS and collected into the 2 mL tube containing the homogenate. During this process, all samples were kept on ice. Samples were transferred to glass extract-O tubes and cortisol was extracted twice with 5 mL of diethyl ether (Fisher Scientific, USA). 3[H]-testosterone was used as a tracer for recovery evaluation, confirming 90% recovery using this protocol. After ether evaporation, the cortisol was reconstituted in 1 mL of 1× PBS. To quantify cortisol concentrations, ELISA was performed using a human salivary cortisol assay kit (Salimetrics LLC, PA). The use of a human salivary cortisol kit to assess whole-body cortisol concentrations in zebrafish was a significant modification of previously published protocols, which typically used much serum cortisol kits [23]. ELISA plates were measured in a VICTOR-WALLAC plate reader and the manufacturer’s software package. Whole-body cortisol levels were determined using a 4-parameter sigmoid minus curve fit based on the absorbances of standardized concentrations and those of the samples. Cortisol levels were further normalized based on the weight of the whole-body sample, and reported as absolute cortisol concentrations (ng/g body weight).
2.7. Statistical analysis
All experimental data was analyzed with a two-sample Wilcoxon U-test for significance between control and experimental groups. The results obtained from manual and automated analysis of zebrafish behavior were further analyzed using Spearman’s rank correlation test. Data is expressed as mean ± SEM. Significance was set at P < 0.05.
3. Results
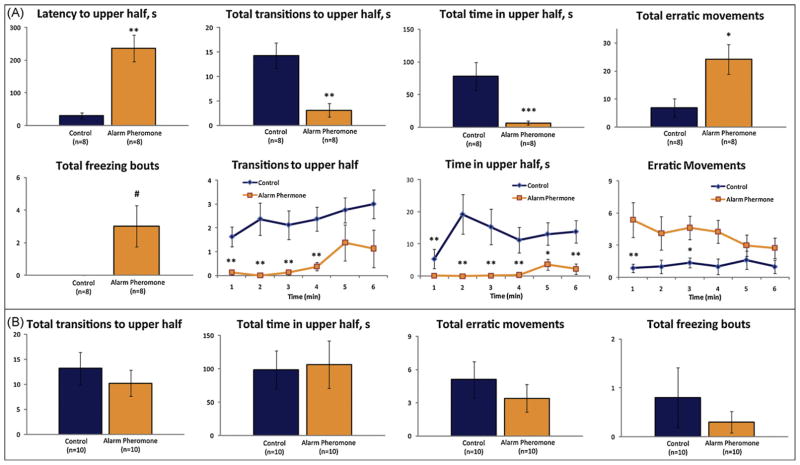
As can be seen in Fig. 2A, acute alarm pheromone exposure resulted in a significantly longer latency to explore the upper portion of the tank, fewer entries and reduced time spent in the top. Alarm pheromone also significantly increased the frequency of erratic movements, with a trend to increased frequency of freezing behavior. In contrast, prolonged alarm pheromone exposure did not result in any significant behavioral differences between the control and experimental groups (Fig. 2B).
Fig. 2.
Anxiogenic effects of alarm pheromone on zebrafish behavior in the novel tank diving test: (A) acute alarm pheromone exposure (7 mL, 6 min). (B) Prolonged alarm pheromone (7 mL, 30 min). Data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.005, #P < 0.09 (trend) vs. control, U-test.
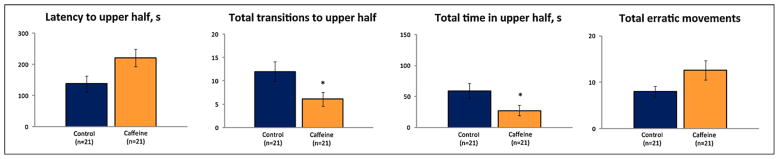
Acute caffeine produced anxiogenic behavioral responses in zebrafish, including a significantly higher latency to enter the upper half, fewer transitions to the upper half, and more erratic movements (Fig. 3), but unaltered freezing behavior (data not shown).
Fig. 3.
Anxiogenic effects of acute caffeine (100 mg/L, 15 min pre-exposure time) on zebrafish behavior in the novel tank diving test. Data are presented as mean ± SEM, * P < 0.05 vs. control, U-test.
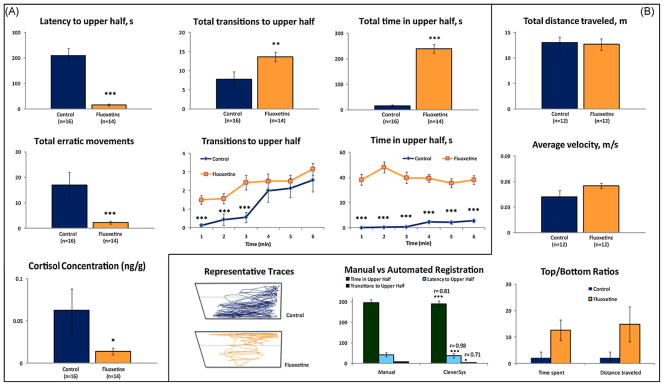
Chronic administration of fluoxetine produced robust effects on zebrafish behavior, including a significantly lower latency to enter the upper half of the novel tank, more time spent in the top, and more transitions to the top. The number of erratic movements was also dramatically reduced in SSRI-treated fish. In parallel to behavioral data, fluoxetine-treated fish also showed significantly lower whole-body cortisol concentrations compared to control group (Fig. 4A).
Fig. 4.
Anxiolytic effects of chronic fluoxetine (100 μg/L, 2 weeks in the home tanks) on zebrafish cortisol levels and behavior in the novel tank diving test. (A) Manual behavioral phenotyping with sample matched cortisol analysis. (B) Automated behavioral characterization with video-tracking software (CleverSys Inc.). Data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.005 vs. control, U-test.
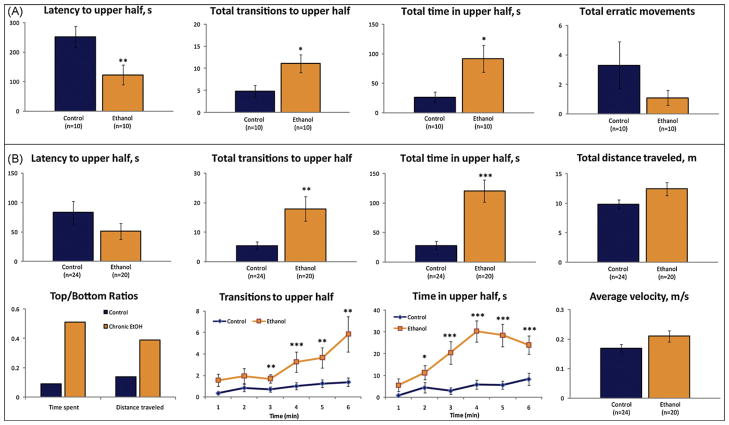
Acute treatment with 0.3% ethanol decreased latency to enter the upper portion of the tank, increased the number of transitions to the upper portion, and increased total time spent in that area (Fig. 5A). Anxiolytic effects were also observed in response to chronic ethanol treatment and included more time spent in top throughout trials (Fig. 5B). In line with this, a separate study using chronic ethanol exposure showed that drug-exposed animals tended to have lower (~30% reduction) levels of cortisol compared to the control group, though this difference was not statistically significant (data not shown).
Fig. 5.
Anxiolytic effects of acute and chronic 0.3% ethanol on zebrafish behavior in the novel tank diving test. (A) Manual behavioral phenotyping (acute ethanol treatment for 5 min). (B) Chronic ethanol (1-week in the home tanks) prior to behavioral analysis with video-tracking software (CleverSys Inc.). Data are presented as mean ± SEM, * P < 0.05, **P < 0.01, ***P < 0.005 vs. control, U-test.
Behavioral assessment of both chronic fluoxetine and ethanol cohorts was performed manually and through automated registration, in order to confirm the validity of the automated behavioral tracking system. For both experiments, automated video-tracking was as reliable as manual registration, as measured by the Spearman correlation coefficient (r > 0.8–0.9; P < 0.00005–0.05) for all endpoints (Figs. 4B and 5B). In both chronic experiments, total distance travelled and average velocity were unaltered (Figs. 4B and 5B), indicating that behavioral differences observed were not accompanied by sedative or toxic side effects of the treatment.
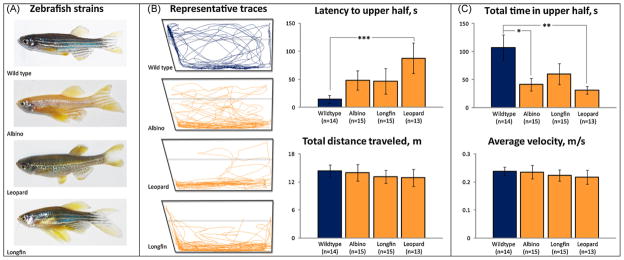
Behavioral strain differences were also observed in this study (Fig. 6). For example, compared to the wild type controls, all three mutant zebrafish strains tended to show longer latencies to enter the top half, although the difference was significant only in the leopard strain. The wild type group also tended to show more entries to the top. Total time spent in the top further highlighted robust differences between the strains (Fig. 6), with the leopard and albino mutant strains spending less time in the top compared with the wild type zebrafish. Importantly, distance travelled and swimming velocity were similar in all four groups, indicating no overt motor/neurological differences in swimming ability between these strains.
Fig. 6.
Strain differences in zebrafish novel tank diving test behavior. Different strains of zebrafish used in this study: (A) display specific patters of their exploratory behavior, as illustrated by representative phenotypic variations in swimming behavior for 3 min, (B) and behavioral endpoints and (C) analyzed using video-tracking software (CleverSys Inc.). *P < 0.05, **P < 0.01, ***P < 0.005 vs. wild type strain, U-test.
4. Discussion
In the present study, zebrafish behavior was observed and quantified in the novel tank diving test, in which manual phenotypic measurements were reconfirmed using video-tracking technology. Overall, behavioral and physiological endpoints measured in the present study (Figs. 2–6) proved to be highly sensitive to environmental and pharmacological challenges. Our experiments also produced several methodological advancements. First, we used a 2-zone “top/bottom” variation of the novel tank (Fig. 1), differing from traditional versions [39,31], which employ three zones (bottom, middle and top). Our results suggest that this simplified protocol does not reduce sensitivity of the novel tank diving paradigm to test a wide spectrum of experimental manipulations, and therefore may be beneficial for phenotyping of zebrafish anxiety.
Second, we have substantially modified the cortisol assay to assess zebrafish stress. While previously published studies used serum cortisol kits, and combined 20–25 fish in order to obtain one cortisol sample (e.g., [5]), we used a more sensitive human salivary cortisol kit, measuring cortisol levels in each individual fish. Such marked increase in sensitivity of whole-body cortisol assay appears to be an important methodological advancement, not only reducing the number of animals per group, but also enabling correlational analyses of behavioral and endocrine endpoints for each individual zebrafish.
Third, we successfully applied a video-tracking system to the novel tank diving test, showing that automated behavioral analysis not only accurately distinguishes between control and experimental animals, but also demonstrates high correlation with data obtained by manual observation (Figs. 4B and 5B). Although the latter measures zebrafish behavior relatively well, intrinsic inter- and intra-rater variability and simple human errors persist. In addition, the presence of experimenters in the testing room may also influence zebrafish behaviors. By reducing these factors, video-tracking method standardizes phenotypic assessment and yields more precise results of higher validity. Video-tracking further improves behavioral analysis because multiple zebrafish can be recorded, subsequently analyzed, and re-analyzed, if necessary. Likewise, this approach assesses previously unavailable endpoints, such as velocity and distance travelled (Fig. 4B) which not only increase data density per experiment but also ensure correct data interpretation. For example, if a drug reduces top exploration but does not affect velocity and total distance travelled, it may be interpreted as a clear anxiogenic action without sedation. Moreover, as the video-tracking system also records the path of movement, it can generate traces (e.g., Fig. 4B) that, in addition to raw data, may reflect zebrafish anxiety phenotypes. Finally, video-tracking method enables rapid batch-processing of behavioral data, again markedly increasing throughput of zebrafish phenotyping.
In general, our data confirm that an unfamiliar environment, anxiogenic drugs, and alarm pheromone each evoke relatively simple, yet robust anxiety-like behavioral responses in zebrafish. Interestingly, prolonged alarm pheromone-exposed zebrafish showed no significant behavioral differences compared to control fish. Thus, alarm pheromone appears to only be effective acutely, reflecting its natural use as a danger signal to nearby shoals.
Zebrafish cohorts acutely exposed to caffeine displayed robust anxiety, evidenced by higher latency to initiate top exploration, fewer transitions to the top, and more erratic movements. The anxiogenic effect seen here in zebrafish is in line with human response to caffeine challenge [40] and rodent data on anxiogenic effects of caffeine [41,42].
Chronic treatment with fluoxetine demonstrates the potential of this model to detect anxiolytic drug response, by inducing an overall increase in time spent in the top portion, lower latency to top exploration, and higher average top transitions. The reduced levels of cortisol seen in the same groups of fluoxetine-treated fish (Fig. 4A) give further support to the efficacy of the zebrafish model to span across levels of analysis. Behavioral and physiological data from this study agree strongly with that seen in previously published in rodent studies showing that chronic exposure to fluoxetine reduces anxiety [43,44], corticosterone responses (mice [44]) and HPA sensitivity (rats [45,46]).
Similar to ethanol-induced anxiolysis in mice [47], acute ethanol exposure produced effects consistent with a reduced anxiety state in zebrafish (Fig. 5). Chronic exposure to ethanol also increased time spent in the upper tank portion, and showed a trend towards reduced cortisol levels (own unpublished data). Collectively, these findings further validate the zebrafish model of anxiety in its behavioral, pharmacological and endocrine aspects.
The reduction in exploratory behavior exhibited by several zebrafish mutant strains (Fig. 6) denotes a greater anxiety response to perceived threats, suggesting a higher baseline anxiety compared to the wild type strain. This observation is important, suggesting genetic differences in anxiety between several commercially available zebrafish strains. These results may have several important implications. For example, researchers must take into consideration robust behavioral differences when using different strains of zebrafish. Likewise, the differing anxiety responses elicited here raises the possibility of expanding this animal model to account for population differences in humans in their susceptibility to stressors. Strain selection could have great applications in experimental design. As some genotypes exhibit higher baseline anxiety levels, selection of a certain strain could improve screening of anxiolytic or anxiogenic compounds. For example, anxiolytic drugs may show more clear-cut effects in anxious zebrafish strains (e.g., leopard strain), whereas less anxious strains (e.g., wild type) may be a better choice for testing anxiogenic drugs and manipulations.
Contemporary phenotype-based whole-organism drug discovery is bottlenecked by mammalian animal models that are expensive, require ample physical space, large quantities of compounds, and exhibit complex behavioral phenotypes that can be difficult to characterize or quantify [1]. Using zebrafish as an animal model provides an effective means to reduce these limiting factors, and in combination with computer-aided video-tracking technologies, makes high-throughput behavioral phenotyping and pharmacological screening a promising avenue of research [1,2,48,49]. While zebrafish appear to have good potential as an animal model of stress, several different domains must be carefully balanced in the observation and interpretation of zebrafish behavior, including (1) locomotion (overall activity), (2) reduction of exploration (anxiety), (3) avoidance behavior, and (4) erratic movements (fear- and/or escape-like behavior). Although the dissection of anxiety vs. fear was not in the scope of this study, detailed analysis of these affected domains may provide insight into the type of stressor applied, and its larger implications.
In summary, we showed robust anxiety-related phenotypes in zebrafish following exposure to experimental stressors and pharmacological agents. These phenotypes were also observed in different strains of zebrafish. We applied video-tracking tools to behavioral analysis, showing high reliability for this method of registration of zebrafish anxiety, also capable of dissecting anxiety responses from overall activity. Finally, we developed a new method of measuring endocrine (cortisol) stress responses that parallel behavioral measures of zebrafish anxiety. Taken together, these results confirm zebrafish as a valid, reliable, and efficacious model for basic translational research of stress-related brain disorders.
Acknowledgments
This work was supported by the NARSAD YI award (to AVK), Georgetown University’s Stress Physiology and Research Center (SPaRC), Tulane University Neuroscience Summer Fellowship (to DHT) and Tulane University intramural research funds.
References
- 1.Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov. 2005;4:35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]
- 2.Shin JT, Fishman MC. From Zebrafish to human: modular medical models. Annu Rev Genomics Hum Genet. 2002;3:311–40. doi: 10.1146/annurev.genom.3.031402.131506. [DOI] [PubMed] [Google Scholar]
- 3.Mueller T, Vernier P, Wullimann MF. The adult central nervous cholinergic system of a neurogenetic model animal, the zebrafish Danio rerio. Brain Res. 2004;1011:156–69. doi: 10.1016/j.brainres.2004.02.073. [DOI] [PubMed] [Google Scholar]
- 4.Panula P, Sallinen V, Sundvik M, Kolehmainen J, Torkko V, Tiittula A, et al. Modulatory neurotransmitter systems and behavior: towards zebrafish models of neurodegenerative diseases. Zebrafish. 2006;3:235–47. doi: 10.1089/zeb.2006.3.235. [DOI] [PubMed] [Google Scholar]
- 5.Alsop D, Vijayan MM. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am J Physiol Regul Integr Comp Physiol. 2008;294:R711–719. doi: 10.1152/ajpregu.00671.2007. [DOI] [PubMed] [Google Scholar]
- 6.Gerlai R, Lee V, Blaser R. Effects of acute and chronic ethanol exposure on the behavior of adult zebrafish (Danio rerio) Pharmacol Biochem Behav. 2006;85:752–61. doi: 10.1016/j.pbb.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borla MA, Palecek B, Budick S, O’Malley DM. Prey capture by larval zebrafish: evidence for fine axial motor control. Brain Behav Evol. 2002;60:207–29. doi: 10.1159/000066699. [DOI] [PubMed] [Google Scholar]
- 8.Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J Neurobiol. 1998;37:622–32. doi: 10.1002/(sici)1097-4695(199812)37:4<622::aid-neu10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 9.Saint-Amant L, Drapeau P. Synchronization of an embryonic network of identified spinal interneurons solely by electrical coupling. Neuron. 2001;31:1035–46. doi: 10.1016/s0896-6273(01)00416-0. [DOI] [PubMed] [Google Scholar]
- 10.Watkins J, Miklosi A, Andrew RJ. Early asymmetries in the behaviour of zebrafish larvae. Behav Brain Res. 2004;151:177–83. doi: 10.1016/j.bbr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Loucks E, Carvan MJ., III Strain-dependent effects of developmental ethanol exposure in zebrafish. Neurotoxicol Teratol. 2004;26:745–55. doi: 10.1016/j.ntt.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Damodaran S, Dlugos CA, Wood TD, Rabin RA. Effects of chronic ethanol administration on brain protein levels: a proteomic investigation using 2-D DIGE system. Eur J Pharmacol. 2006;547:75–82. doi: 10.1016/j.ejphar.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Airhart MJ, Lee DH, Wilson TD, Miller BE, Miller MN, Skalko RG. Movement disorders and neurochemical changes in zebrafish larvae after bath exposure to fluoxetine (PROZAC) Neurotoxicol Teratol. 2007;29:652–64. doi: 10.1016/j.ntt.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Barbazuk WB, Korf I, Kadavi C, Heyen J, Tate S, Wun E, et al. The syntenic relationship of the zebrafish and human genomes. Genome Res. 2000;10:1351–8. doi: 10.1101/gr.144700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dooley K, Zon LI. Zebrafish: a model system for the study of human disease. Curr Opin Genet Dev. 2000;10:252–6. doi: 10.1016/s0959-437x(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 16.Ernest S, Rauch GJ, Haffter P, Geisler R, Petit C, Nicolson T. Mariner is defective in myosin VIIA: a zebrafish model for human hereditary deafness. Hum Mol Genet. 2000;9:2189–96. doi: 10.1093/hmg/9.14.2189. [DOI] [PubMed] [Google Scholar]
- 17.Goldsmith P, Harris WA. The zebrafish as a tool for understanding the biology of visual disorders. Semin Cell Dev Biol. 2003;14:11–8. doi: 10.1016/s1084-9521(02)00167-2. [DOI] [PubMed] [Google Scholar]
- 18.Bass SL, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: the effects of sympatric and allopatric predators and harmless fish. Behav Brain Res. 2008;186:107–17. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 19.Engeszer RE, Ryan MJ, Parichy DM. Learned social preference in zebrafish. Curr Biol. 2004;14:881–4. doi: 10.1016/j.cub.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 20.Pyron M. Female preferences and male–male interactions in zebrafish (Danio rerio) Can J Zool. 2003;81:122–5. [Google Scholar]
- 21.Mann KD, Turnell ER, Atema J, Gerlach G. Kin recognition in juvenile zebrafish (Danio rerio) based on olfactory cues. Biol Bull. 2003;205:224–5. doi: 10.2307/1543264. [DOI] [PubMed] [Google Scholar]
- 22.Vitebsky A, Reyes R, Sanderson MJ, Michel WC, Whitlock KE. Isolation and characterization of the laure olfactory behavioral mutant in the zebrafish Danio rerio. Dev Dyn. 2005;234:229–42. doi: 10.1002/dvdy.20530. [DOI] [PubMed] [Google Scholar]
- 23.Barcellos LJG, Ritter F, Kreutz LC, Quevedo RM, Bolognesi da Silva L, Bedin AC, et al. Whole-body cortisol increases after direct and visual contact with a predator in zebrafish Danio rerio. Aquaculture. 2007;272:774–8. [Google Scholar]
- 24.Darland T, Dowling JE. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc Natl Acad Sci USA. 2001;98:11691–6. doi: 10.1073/pnas.191380698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kily LJ, Cowe YC, Hussain O, Patel S, McElwaine S, Cotter FE, et al. Gene expression changes in a zebrafish model of drug dependency suggest conservation of neuro-adaptation pathways. J Exp Biol. 2008;211:1623–34. doi: 10.1242/jeb.014399. [DOI] [PubMed] [Google Scholar]
- 26.Ninkovic J, Bally-Cuif L. The zebrafish as a model system for assessing the reinforcing properties of drugs of abuse. Methods. 2006;39:262–74. doi: 10.1016/j.ymeth.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000;20:9187–94. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colwill RM, Raymond MP, Ferreira L, Escudero H. Visual discrimination learning in zebrafish (Danio rerio) Behav Process. 2005;70:19–31. doi: 10.1016/j.beproc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Williams FE, White D, Messer WS. A simple spatial alternation task for assessing memory function in zebrafish. Behav Processes. 2002;58:125–32. doi: 10.1016/s0376-6357(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 30.Blaser R, Gerlai R. Behavioral phenotyping in zebrafish: comparison of three behavioral quantification methods. Behav Res Methods. 2006;38:456–69. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
- 31.Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol Behav. 2007;90:54–8. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Von Frisch K. Über einen Schreckstoff der Fischhaut und seine biologiche Bedeutung. Z Vergl Physiol. 1941:46–145. [Google Scholar]
- 33.Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav Brain Res. 2008;188:168–77. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehnberg BG, Smith RJF. The influence of alarm substance and shoal size on the behaviour of zebra danios, Brachydanio rerio (Cyprinidae) J Fish Biol. 1988;33:155–63. [Google Scholar]
- 35.Peitsaro N, Kaslin J, Anichtchik OV, Panula P. Modulation of the histaminergic system and behaviour by alpha-fluoromethylhistidine in zebrafish. J Neurochem. 2003;86:432–41. doi: 10.1046/j.1471-4159.2003.01850.x. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Patino MA, Yu L, Cabral H, Zhdanova IV. Anxiogenic effects of cocaine withdrawal in zebrafish. Physiol Behav. 2008;93:160–71. doi: 10.1016/j.physbeh.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Alsop D, Vijayan M. The zebrafish stress axis: Molecular fallout from the teleost-specific genome duplication event. Gen Comp Endocrinol. 2008 doi: 10.1016/j.ygcen.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Alderman SL, Bernier NJ. Ontogeny of the corticotropin-releasing factor system in zebrafish. Gen Comp Endocrinol. 2009 doi: 10.1016/j.ygcen.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Bencan Z, Levin ED. The role of alpha7 and alpha4beta2 nicotinic receptors in the nicotine-induced anxiolytic effect in zebrafish. Physiol Behav. 2008;95:408–12. doi: 10.1016/j.physbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Childs E, Hohoff C, Deckert J, Xu K, Badner J, de Wit H. Association between ADORA2A and DRD2 polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2008;33:2791–800. doi: 10.1038/npp.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM. The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A(2A) adenosine receptor antagonists. Psychopharmacology (Berl) 2000;148:153–63. doi: 10.1007/s002130050037. [DOI] [PubMed] [Google Scholar]
- 42.Sudakov SK, Medvedeva OF, Rusakova IV, Figurina IB. Effect of short-term and chronic caffeine intake on rats with various anxiety level. Bull Exp Biol Med. 2001;132:1177–9. doi: 10.1023/a:1014511415473. [DOI] [PubMed] [Google Scholar]
- 43.Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–30. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- 44.Norcross M, Mathur P, Enoch AJ, Karlsson RM, Brigman JL, Cameron HA, et al. Effects of adolescent fluoxetine treatment on fear-, anxiety- or stress-related behaviors in C57BL/6J or BALB/cJ mice. Psychopharmacology (Berl) 2008;200:413–24. doi: 10.1007/s00213-008-1215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lowry CA, Hale MW, Plant A, Windle RJ, Shanks N, Wood SA, et al. Fluoxetine inhibits corticotropin-releasing factor (CRF)-induced behavioural responses in rats. Stress. 2009;12:225–39. doi: 10.1080/10253890802309861. [DOI] [PubMed] [Google Scholar]
- 46.Szymanska M, Budziszewska B, Jaworska-Feil L, Basta-Kaim A, Kubera M, Leskiewicz M, et al. The effect of antidepressant drugs on the HPA axis activity, glucocorticoid receptor level and FKBP51 concentration in prenatally stressed rats. Psychoneuroendocrinology. 2009;34:822–32. doi: 10.1016/j.psyneuen.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Houchi H, Warnault V, Barbier E, Dubois C, Pierrefiche O, Ledent C, et al. Involvement of A2A receptors in anxiolytic, locomotor and motivational properties of ethanol in mice. Genes Brain Behav. 2008;7:887–98. doi: 10.1111/j.1601-183x.2008.00427.x. [DOI] [PubMed] [Google Scholar]
- 48.McGrath P, Li CQ. Zebrafish: a predictive model for assessing drug-induced toxicity. Drug Discov Today. 2008;13:394–401. doi: 10.1016/j.drudis.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Rubinstein AL. Zebrafish assays for drug toxicity screening. Expert Opin Drug Metab Toxicol. 2006;2:231–40. doi: 10.1517/17425255.2.2.231. [DOI] [PubMed] [Google Scholar]