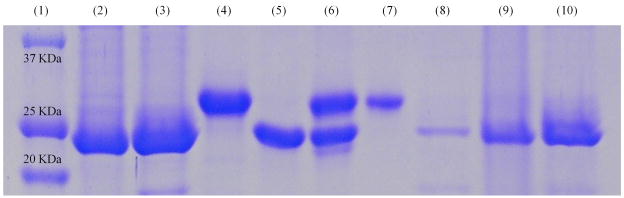
Figure 3.
SDS-PAGE analysis of the affinity capture-elution assay performed with the selected SICLOPPS hits. The unspliced constructs containing sequences CIFYYV and CDLRWF were immobilized via chitin-binding domain (CBD) fusion fragments on chitin beads and incubated with an equimolar mixture of Hdm2 or Hdmx (1 mM). Retained materials were subsequently treated with a solution (1 mM) of a p53-derived peptide (ETFSDLWKLL), and the eluate as well as other components of the assay were analyzed by SDS-PAGE. The lane assignments are as follows: Lane 1 is a protein ladder; Lanes 2 and 3 correspond to the CIFYYV and CDLRWF SICLOPPS constructs, respectively, isolated by chitin beads from crude overexpression lysates; Lanes 4 and 5 contain purified Hdm2 and Hdmx, respectively; Lane 6 corresponds to an equimolar mixture of Hdm2 and Hdmx; Lanes 7 and 8 contain protein material eluted with the p53-derived peptide from the affinity supports containing CIFYYV and CDLRWF leads, respectively, which were pretreated with the equimolar mixture of Hdm2 and Hdm; Lanes 9 & 10 were loaded with the post-elution material retained by the chitin beads pre-functionalized with the CIFYYV and CDLRWF constructs, respectively.