Abstract
Plasma nitrite is a major oxidation product of nitric oxide. It has also recently been suggested to perform an endocrine-like function as a nitric oxide donor in hypoxic tissues, allowing vasodilation. Exercise performance is limited in peripheral arterial disease due to an inadequate blood supply to working tissues. We hypothesized that exercise training in peripheral arterial disease subjects will demonstrate improved “plasma nitrite flux” and endothelial function, to accompany increased exercise performance. Peripheral arterial disease subjects were tested at baseline and following 3 months supervised or home exercise training.
Venous blood (arm) was drawn at rest and 10min following a maximal graded treadmill test. Samples were added to heparin, centrifuged and plasma snap frozen for analysis by reductive chemiluminescence. Brachial artery endothelial function was measured in response to a hyperemic stimulus (flow-mediated dilation). At 3 months the peripheral arterial disease-supervised exercise group showed increases in claudication onset pain time (+138sec, p≤0.05) peak walking time (+260sec, p≤0.01), VO2peak (1.3ml/kg/min, p≤0.05), brachial artery flow-mediated dilation (+2%, p≤0.05) and plasma nitrite flux (+33% p≤0.05). There were no changes in the peripheral arterial disease-home exercise group. The change in plasma nitrite flux predicted the change in claudication onset pain (r2=0.59, p≤0.01).
These findings suggest changes in plasma nitrite are related to endothelial function and predict exercise performance in peripheral arterial disease.
Keywords: Endothelium, Nitric Oxide, Plasma Nitrite, Peripheral Vascular Disease, Exercise, VO2peak, intermittent claudication
INTRODUCTION
Peripheral artery disease is a form of cardiovascular disease caused by atherosclerotic occlusions in the legs and affects approximately 5% of the US population over 50yrs of age1. Intermittent claudication is the main manifestation of peripheral arterial disease where there is a failure to adequately supply oxygen and other nutrients to skeletal muscle during walking which subsides with rest2. Dysfunction of endothelial cells is an early event in the process of atherosclerotic lesion formation3, and is associated with risk factors for cardiovascular disease 4–6.
The key mediator of endothelial function is the bioavailability of nitric oxide (NO), which modulates blood flow, decreases platelet aggregation, diminishes cellular vascular adhesion and is anti-atherogenic.7 The short half-life and rapid metabolism of NO in the blood make it impractical for direct measurement and have led to the use of other nitrogen species as surrogates for NO. The most viable of these is venous plasma nitrite which has a low background concentration and is relatively stable. In fact, plasma nitrite has previously been suggested to be an “NO-sink” that reflects acute changes in regional vascular NO production following both chemical (l-arginine and NG-monomethyl-L-arginine8, and physiological (hyperemia) 8, 9 stimuli. It is estimated approximately 70% of resting plasma nitrite is derived from eNOS activity in humans and other mammalian species10.
The discovery of endocrine roles for NO equivalents in controlling hypoxic vasodilation11–14 have led to a search for particular NO metabolites that can control vascular function. One such metabolite is nitrite. Rather than just an “NO-sink”, plasma nitrite has now been proposed to be transported in the circulation to be converted back to NO under hypoxic conditions15, 16. This suggests a “push-pull” model for plasma nitrite whereby during normoxia localized vascular NO production leads to plasma nitrite concentration increases (via NO oxidation), whereas during hypoxia (when eNOS is dysfunctional) nitrite can be reduced back to NO to cause vasodilation. This complementary system enables NO to be available to vessels across the oxygen gradient. It also raises the potential for the measurement of relative changes (or flux) in plasma nitrite to represent a dynamic index of NO bioavailability and vascular health. Patients with atherosclerotic occlusions in the legs that limit oxygen supply to peripheral tissues and produce physical manifestations of ischemic pain (claudication) during exercise may be an ideal population to investigate these markers.
We have previously shown, in a cross sectional approach, that “plasma nitrite flux” in response to acute exercise stress, was the most powerful predictor of exercise performance, followed by brachial artery flow-mediated dilation (an indices of vascular endothelial function), in subjects with risk factors for or with established peripheral arterial disease 17. In several other studies exercise training has been shown to delay the time to claudication onset pain and improve exercise performance in peripheral arterial disease subjects18. One other previous study has shown increases in endothelial function19.
To date no studies have examined the effects of chronic exercise training on “plasma nitrite flux” response to acute exercise stress in peripheral arterial disease. If plasma nitrite flux is a good marker for NO production and, hence, endothelial function, and supervised exercise improves endothelial function in patients with peripheral arterial disease, we would expect these patients who undergo SE to have increases in plasma nitrite flux. Therefore, the hypothesis of this study is that following 3 months supervised exercise training subjects with peripheral arterial disease will demonstrate improved endothelial function and “plasma nitrite flux”, accompanying increased exercise performance. Subjects receiving standard care (including instructions to exercise at home) will show no changes in any of the measures.
MATERIALS AND METHODS
Patient Characteristics
This study is a sub-study of the larger Angiogenesis and Mechanisms of Exercise Training in Peripheral Arterial Disease (AMNESTI) study. The full study was conducted at Duke University Medical Center and The University of Colorado School of Medicine at Denver. For this sub-study, subjects from the Duke University Medical Center site that completed the baseline and 3 month visits, exercise training and consented to undergo the additional testing procedures were included. Prior to participation all subjects signed an informed consent document approved by Duke University Medical Center Internal Review Board. All subjects were aged 40 to 75 years.
Peripheral arterial disease subjects had a history of stable intermittent claudication for 3 or more months and an ankle-brachial pressure index <0.9 at rest (ankle-brachial index is the ratio of blood pressure in the lower legs to the blood pressure in the arms, a ratio of <0.9 is considered a diagnosis of peripheral arterial disease). These subjects were all non-diabetic and receiving anti-platelet and lipid lowering therapy unless medically contraindicated by their physician. Exclusions were based on a past medical history of gangrene, impending limb loss or osteomyelitis, lower extremity vascular surgery, angioplasty or lumbar sympathectomy within 3 months of enrollment, severe peripheral neuropathy, any condition other than peripheral arterial disease that limits walking, unstable angina, history of significant left main disease or three vessel coronary artery disease (>70% stenosis, unprotected by grafts) or recent myocardial infarction (6 weeks), chest pain during treadmill exercise which appears before the onset of claudication, or >3 mm ST depression during exercise.
Subjects free from symptomatic coronary artery disease, an ankle-brachial index of > 1.0, no complicating illnesses, with resting systolic blood pressure < 170 and resting diastolic < 100, and not actively enrolled in an exercise program were used as baseline controls.
Study Design
The control subjects were used as a control population for baseline comparisons where applicable. The peripheral arterial disease subjects were tested at baseline then randomized to the supervised exercise or home exercise group. Repeat testing was performed following 3 months for both groups (the supervised exercise group only had an additional 3 weeks testing time point -data not included here).
The supervised exercise protocol was based on published optimal programs for improving claudication pain distances in patients with peripheral arterial disease 18, 20. Exercise training was performed 3 times per week for 3 months. All sessions were supervised by a trained exercise physiologist. Subjects walked until claudication pain became moderately severe, at which time they stepped off the treadmill and rested until claudication pain subsided. Exercise and rest periods were repeated during each training session until a total of 30 to 40 minutes of walk time was achieved. The initial training intensity was set to the workload that brought on claudication pain during the maximal treadmill test performed at baseline. In subsequent visits, the speed and/or elevation was increased once the subject could walk for greater than 8–10 minutes or longer without reaching moderate pain. Subjects randomized to the usual care arm were prescribed a home based exercise program (walking 3 × week for 30 minutes) which is the medical standard of care in the peripheral arterial disease population. These subjects were asked to keep careful notes regarding their activity and were called once every three weeks to answer any exercise related questions. It has previously been shown that the home exercise program has little effect on exercise capacity or related quality of life measurements21.
Measures of Exercise Performance
All subjects performed maximal cardiopulmonary exercise testing on a treadmill with gas exchange analysis and a 12-lead electrocardiograph. Expired gases were analyzed continuously using a ParvoMedics unit (Sandy UT, USA) and averaged in 15-second intervals. Subjects were encouraged to walk until they could no longer continue (Peak Walking Time). The Gardner protocol, which maintains 2mph with a 2% grade increase every 2 minutes was used for the peripheral arterial disease subjects. The time to claudication onset pain was also recorded in this group. The Gardner protocol is specifically designed for a claudication-limited peripheral arterial disease population and therefore, an alternative protocol was necessary to evaluate maximal functional capacity in the control group. The Storey protocol which increases in workload by approximately 1 MET per each 2 minute stage was used for these subjects (preventing comparisons for peak walking time between control and peripheral arterial disease groups). Blood pressure measurements and ratings of perceived exertion were also obtained at the end of each minute throughout the test.
Arterial Vasoreactivity Measures
Prior to imaging (on a separate day from cardiopulmonary exercise testing), subjects were instructed to hold medications, fast and refrain from exercise for 12hr and alcohol for 48hr. All vascular imaging and ankle-brachial index measures were performed between 8am and 11am with the subject in a supine position. Brachial artery assessments were obtained on the left arm, with the forearm extended and slightly supinated using high resolution ultrasound and a 7.5MHz linear array transducer (Accuson, Sequoia 512). Measures were taken at baseline (following 10min of supine rest), during five minutes of forearm occlusion, and continuously on r-wave trigger for 2 minutes following cuff release (hyperemia) (for further details and reproducibility data see 22, 23). The percent change in brachial artery diameter was calculated by…
((peak post-hyperemia diastolic diameter – baseline diastolic diameter)/baseline diastolic diameter)*100
Following an additional 15 minutes rest, endothelium independent 0.4mg nitroglycerine-mediated) dilation was measured.
Nitric Oxide Metabolite Measures
Prior to initiation of the cardiopulmonary exercise test, a 20gauge I.V. catheter was placed in the cephalic vein. Approximately, five ml of blood was taken prior to (Pre), and within 10 minutes of the exercise test termination (Post). Samples were separated into 1ml eppendorf tubes containing 5uL heparin (1 to 1000U/ml) and centrifuged at 5000g for 1 minute. Plasma samples were then removed into separate tubes, snap-frozen in liquid nitrogen and stored at −70°C until analysis.
All nitric oxide metabolite concentrations were measured (within 30mins of defrosting) by chemiluminescence using Ionics/Sievers nitric oxide analyzer (NOA 280), as per manufacturer’s instructions (Sievers Instruments, Boulder, CO). The reductant used for nitrite analysis was potassium iodide in acetic acid, which has the reduction potential to convert nitrite to nitric oxide but is insufficient to reduce any higher oxides of nitrogen such as nitrate and thus is relatively specific for nitrite. To obtain concentrations of total plasma nitrogen oxides we used the same apparatus with a stronger reductant, vanadium chloride in hydrochloric acid at 94°C. This stronger reductant reduces the sum of all nitrogen oxides with an oxidation state of +2 or higher which is predominantly nitrate [µM] but also includes both nitrite [nM] and nitrosothiols [nM].
Statistics
All statistical analyses were performed using SPSS for Windows (version 15.0). Baseline differences between groups were examined with one-way analysis of variance with Túkey post-hoc analysis. Additionally, an analysis of covariance adjusting for age was used to identify group differences for brachial artery flow mediated dilation and VO2peak. Fisher’s exact test was used to calculate differences between groups for categorical variables.
In order to determine if the supervised exercise or home exercise groups changed significantly over time, repeated measures t-tests were used within each group to compare the levels of the following variables at baseline and 3 months:- nitric oxide metabolites (“plasma nitrite flux” and plasma nitrate markers), endothelial function (brachial artery flow-mediated dilation), ankle-brachial index, and functional performance (claudication onset pain, peak walking time and VO2peak). Pearson product moment correlations were used to examine univariate relations between variable change scores. An alpha level of p≤0.05 was required for statistical significance.
RESULTS
Patient Characteristics
At baseline the analysis included 41 control and 35 peripheral arterial disease subjects for vascular and exercise testing measures. Of these subjects 21 controls and 28 peripheral arterial disease subjects provided blood samples for nitric oxide metabolite analysis. The pre-randomization baseline subject characteristics are summarized in table 1. The control group was younger, had a higher fitness level, lower resting systolic blood pressure and, as expected, higher ankle-brachial index values than the peripheral arterial disease group. There were no differences in resting diastolic blood pressures or brachial artery diameters between groups. Prior to testing subjects were not placed on a nitrate controlled diet.
Table 1.
Subject baseline characteristics.
| CON (n=41, NO=21) |
PAD (n=35, NO=28) |
p-value | |
|---|---|---|---|
| Age (yr) | 53±1 | 67±2 | ≤0.01 |
| Ht (cm) | 169±2 | 172±3 | NS |
| Wt (kg) | 80±3 | 78±3 | NS |
| BMI | 28.6±0.9 | 27.8±1.0 | NS |
| SBP (mmHg) | 130±3 | 156±4 | ≤0.01 |
| DBP (mmHg) | 81±2 | 80±2 | NS |
| ABI | 1.05±0.02 | 0.66±0.04 | ≤0.01 |
| VO2peak (ml/kg/min) | 24.0±0.9 | 16.6±0.8 | ≤0.01 |
| COT (sec) | N/A | 248±35 | |
| Brach Diam (mm) | 3.2±0.1 | 3.3±0.1 | NS |
| Rest Plasma NO2− (nM) | 94±12 | 115±12 | NS |
| Rest Plasma NO3− (µM) | 14±2 | 26±4 | ≤0.01 |
| CAD (%) | 0 | 41 | ≤0.05 |
| CVA (%) | 0 | 22 | ≤0.05 |
| CHF (%) | 0 | 13 | ≤0.05 |
| Smoking % | |||
| Never | 74 | 22 | ≤0.05 |
| Former | 19 | 47 | ≤0.05 |
| Current | 7 | 31 | ≤0.05 |
| Medications % | |||
| Ace Inhibitor | 2 | 41 | ≤0.05 |
| Beta Blocker | 0 | 41 | ≤0.05 |
| Statin | 12 | 72 | ≤0.05 |
| Aspirin | 26 | 69 | ≤0.01 |
CON = Controls, PAD = Peripheral Arterial Disease Subjects, NO = number of subjects for nitric oxide metabolite analysis, ABI = Ankle-Brachial Index, COT = Claudication Onset Time, CAD = Coronary Artery Disease, CVA = Cerebrovascular Disease, CHF = Congestive Heart Failure
Following baseline randomization (and study intervention completion with repeat testing) we had 15 peripheral arterial disease subjects in the supervised exercise group (14 for nitric oxide metabolite analysis) and 18 in the home exercise instruction group (14 for nitric oxide analysis). There were no differences between these two groups at baseline (immediately after randomization).
Exercise Capacity
At baseline the control group had a greater VO2peak than the peripheral arterial disease home exercise or peripheral arterial disease supervised exercise groups (24.6±1.0ml/kg/min v 17.6±1.2ml/kg/min and 15.6±0.8ml/kg/min respectively; p≤0.01). Just as there were no differences in VO2peak between the two peripheral arterial disease groups at baseline, there were similarly no differences in claudication onset time (179±30 v 168±32sec) or peak walking time (623±94 and 504±59sec) between the peripheral arterial disease home exercise and peripheral arterial disease supervised exercise groups.
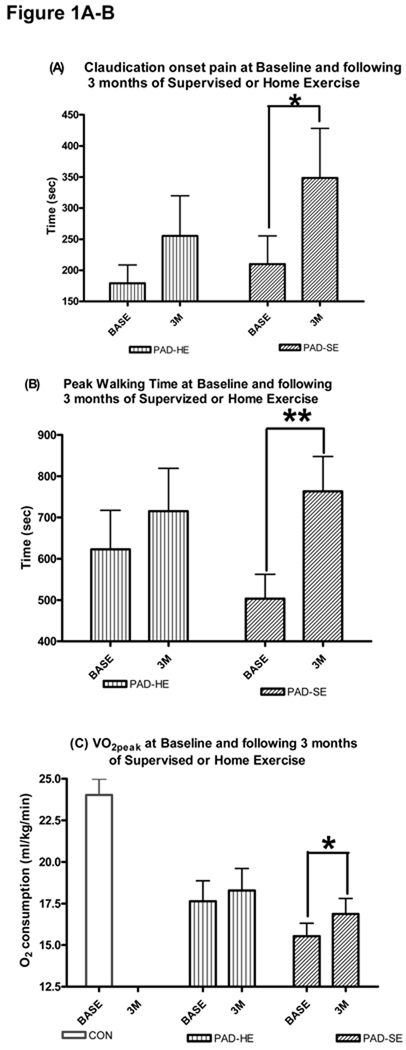
Following 3 months of intervention there were no significant changes in claudication onset time (256±64sec), peak walking time (716±104sec), or VO2peak (18.2±1.2ml/kg/min) for the PAD-HE group. The peripheral arterial disease supervised exercise group, however, increased claudication onset time by Δ138sec (p≤0.05; see figure 1a), peak walking time by Δ260sec (p≤0.01; see figure 1b), and VO2peak by Δ1.3ml/kg/min (p≤0.05: see figure 1c) from baseline values.
Figure 1.
A–C:- Exercise Performance Measures at Baseline and following 3 months of Supervised or Home Exercise. Changes in (A) Time to Claudication Onset Pain (sec), (B) Peak Walking Time (sec), and (C) VO2peak (ml/kg/min) from baseline (BASE) to 3 months post (3M) supervised exercise training (SE) or instructions to exercise at home (HE). Values are mean±SE. * = p≤0.05, ** =. p≤0.01. PAD = peripheral arterial disease subjects, CON = Controls
Arterial Vasoreactivity
There were no differences in resting brachial artery diameters between groups at baseline (see table 1) or following 3 months of supervised or home exercise (data not shown). All groups significantly increased brachial diameters (mm) in response to both flow stimulus and sublingual nitroglycerine, as shown by within group paired t-test analysis.
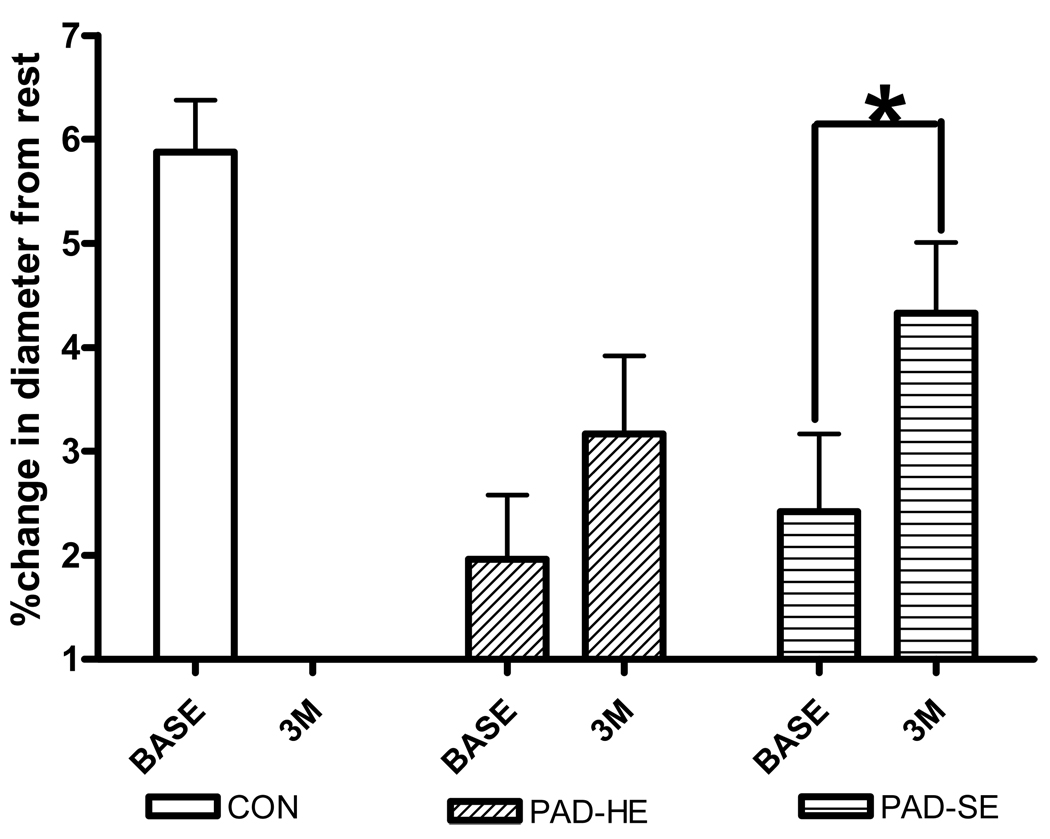
At baseline, the peak percent dilation response to hyperemia (brachial artery flow-mediated dilation) for the control group (5.9±0.6%) was significantly greater than for the peripheral arterial disease home exercise (2.1±0.5%) or peripheral arterial disease supervised exercise group (2.4±0.8%) (p≤0.01) (see figure 2). These differences remained after statistically adjusting for age. There were no significant differences in blood flow velocities or calculated volumes between the groups at any stage in the protocol indicating a similar vasodilatory stimulus for all groups.
Figure 2.
Brachial Artery Flow-Mediated Dilation at Baseline (BASE) and following 3 months (3M) of supervised (SE) or home exercise (HE). Values are mean±SE. * = p≤0.05. PAD = peripheral arterial disease subjects, CON = Controls
Following 3 months of intervention there were no changes in ankle-brachial index for any of the groups (data not shown). There was also no statistical change in brachial artery flow-mediated dilation from pre-intervention for the peripheral arterial disease home exercise group (3.2±0.8%). The peripheral arterial disease supervised exercise group, however, increased brachial artery flow-mediated dilation by Δ1.9% versus pre-intervention (p≤0.05; see figure 2).
Nitric Oxide Metabolites
At the baseline visit there were no differences in resting plasma nitrite values between groups (see table 1 and figure 3a). Between group t-test analysis revealed a significant difference between baseline plasma nitrate values for control vs peripheral arterial disease (p≤0.01) (see table 1). This difference cannot be explained at this time but, given there were within group differences in plasma nitrate during cardiopulmonary exercise testing it suggests this did not impact the plasma nitrite flux measures in this study (data not shown).
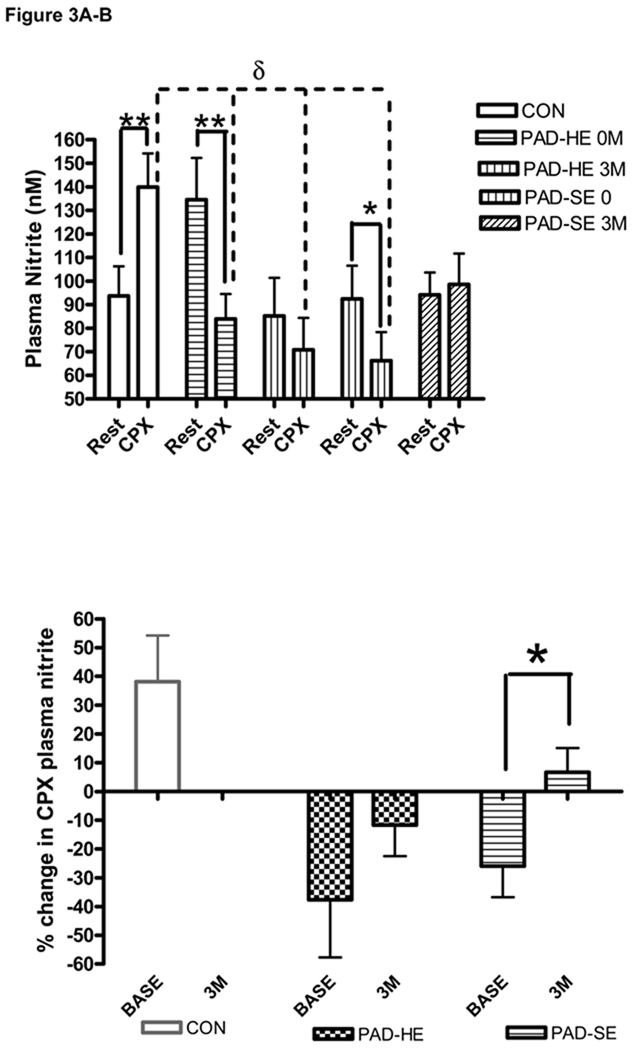
Figure 3.
A–B:- Plasma Nitrite prior to and following a maximal Cardiopulmonary Exercise Testing (CPX). Changes in (A) Circulating plasma nitrite concentration (nM) and (B) plasma nitrite flux (%change in plasma nitrite concentration for pre to post CPX) for both baseline (0M) and 3 month (3M) visits. Samples were collected prior to (Rest), and 10 min following CPX (CPX). * = significantly different within groups at the p≤0.05 level. ** = significantly different within groups at the p≤0.01 level. δ = significantly different between groups at the p≤0.05 level. PAD = peripheral arterial disease subjects, CON = Controls, SE = supervised exercise training, HE = instructed to exercise at home
However, following the baseline cardiopulmonary exercise testing ANOVA analysis revealed the control group had significantly greater plasma nitrite concentrations than the other groups (after adjustment for both age and VO2peak)17. Furthermore, within group (pre-post) t-test analysis showed a significant increase in “plasma nitrite flux” in the control group (+49%) and a significant decrease in the peripheral arterial disease home exercise (−38%) and peripheral arterial disease supervised exercise (−28%) groups (see figure 3a).
Following 3 months of peripheral arterial disease home exercise intervention the post cardiopulmonary exercise testing plasma nitrite level was also still significantly lower than the control group by ANOVA analysis (p≤0.05) (see figure 3a). Additionally, “plasma nitrite flux” for this group remained negative (−11.7±10.8%) and was not significantly different from pre-training (see figure 3b).
In the peripheral arterial disease supervised exercise group however, there was no longer a significant difference between post cardiopulmonary exercise testing plasma nitrite level at 3 months and control values (see figure 3a). Additionally, “plasma nitrite flux” became positive (+6.6±8.5%, p<0.05) (see figure 3b).
Relationships between Plasma Nitrite, Endothelial Function and Exercise Performance
At baseline there were several univariate relationships between each of the primary dependent variables. Plasma “nitrite flux” during cardiopulmonary exercise testing (ΔnM) was significantly correlated with both whole body exercise capacity (VO2peak) (r2= 0.12, p≤0.05) and localized endothelial function measured by brachial artery flow-mediated dilation (% change) (r2= 0.18, p≤=0.01). There were also a significant correlation between brachial artery flow-mediated dilation and VO2peak (r2=0.14, p≤0.05). As expected for the peripheral arterial disease group claudication onset pain time was highly correlated to VO2peak (r2=0.43, p≤0.01).
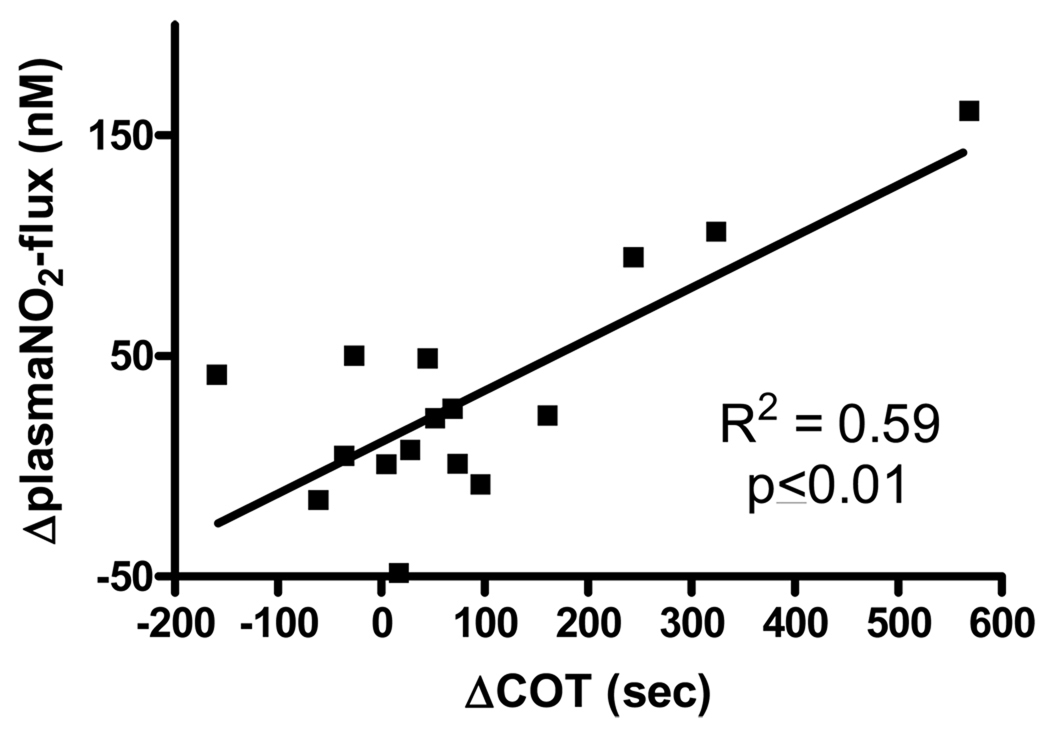
Following the intervention the Δ claudication onset pain (from baseline to 3M) was significantly related to the Δplasma “nitrite flux” for both the peripheral arterial disease home exercise (r2=0.61, p≤0.05) and peripheral arterial disease supervised exercise (r2=0.55, p≤0.05) groups individually and combined (see figure 4).
Figure 4.
Relationship between the Change in Time to Claudication Onset Pain (COT) and Change in Plasma Nitrite Flux in Peripheral Arterial Disease subjects following 3 months of study intervention.
DISCUSSION
The major findings of this study are that following 3 months of supervised exercise training, subjects with clinically diagnosed vascular disease demonstrate statistically significant increases in; a) endothelial function (brachial artery flow-mediated dilation) and b) “plasma nitrite flux”. These changes accompany the expected increase in exercise performance (claudication onset pain, peak walking time, and VO2peak). Most significantly the degree of change in onset of limb ischemia (claudication onset pain) is significantly related to the change in cardiopulmonary exercise testing “plasma nitrite flux” - a potential measure of nitric oxide bioavailability across the peripheral arterial disease population. We have previously shown these physical, physiological, and biochemical indicators of vascular health/function to be interrelated at baseline17. The home exercise group demonstrated no significant changes from pre-intervention for any of the measures of nitric oxide metabolites, endothelial function or exercise performance. These post training findings are unique and provide an important insight into the role of plasma nitrite in endothelial dysfunction and vascular disease. It is noteworthy that there are non-significant trends towards improvements in the home exercise group for each of the variables measured. This can be attributed to the variable response of subjects to the exercise at home instructions. Some subjects increase physical activity when instructed to do so by a physician, unfortunately, most apparently do not. This is not a new finding, other studies have shown home exercise to be ineffective in increasing exercise capacity or related quality of life measurements in peripheral arterial disease.21
Exercise Capacity
Peripheral arterial disease with intermittent claudication is a manifestation of atherosclerosis in the arteries that supply the leg and feet. Stenosis or occlusion of the vessel lumens creates a situation in which regional blood flow is unable to meet the demands of working skeletal muscle, and results in claudication pain. When exercise stops and blood flow demand falls this pain is resolved. In most clinical studies the most commonly measured way to determine the severity of peripheral arterial disease are ankle-brachial index and exercise performance including time to claudication onset pain. It is clear from numerous studies that exercise training is a powerful rehabilitative measure for those with established peripheral arterial disease 18, 24–28. Furthermore the average improvement in exercise performance is near 100% across studies with time to claudication onset pain as high as 130%29. In the current study we demonstrated increases in exercise performance in the supervised exercise group, with a magnitude of 66% for time to claudication onset pain, 52% for peak walking time, and 9% for VO2peak. There were no changes in resting ankle-brachial index values for any of the treatment groups, suggesting the degree of limb stenosis/occlusion at rest remained similar. The contribution of increases in skeletal muscle capillary density and oxidative machinery to these increases in function are currently under investigation.
Arterial Vasoreactivity
In peripheral arterial disease populations brachial artery flow-mediated dilation has been shown to be an independent predictor of long-term cardiovascular events and adds to the prognostic value of the ankle-brachial index (currently the most powerful prognostic indicator in peripheral arterial disease)30, 31. Other have shown a relationship between brachial artery flow-mediated dilation and physical activity levels in peripheral arterial disease subjects32. We have previously shown in a cross-sectional study a graded brachial artery flow-mediated dilation response, able to distinguish among control subjects, type 2 diabetic and peripheral arterial disease populations17.
Our current findings extend this work to show increases in brachial artery flow-mediated dilation following 3 months of SE but no changes following home exercise. This is similar to the findings of Brendle et al., who in a single arm prospective study, showed an increase in brachial artery flow-mediated dilation from 4.8±0.8% to 8±1% in 19 peripheral arterial disease subjects following 6 months of a supervised rehabilitation program (along with a 94% increase in claudication onset pain time)19. We found no differences in resting arterial diameters, hyperemic blood flow velocities or volumes’, suggesting the stimulus signal for dilation was similar for all groups. Additionally, smooth muscle function in response to sub-lingual nitroglycerine was similar between groups, suggesting that this process is dependent upon increased endothelial nitric oxide bioavailability.
Overall this indicates an up regulation in vascular endothelial nitric oxide production following chronic exercise training in peripheral arterial disease subjects and is consistent with the brachial artery flow-mediated dilation responses shown in other cardiovascular disease populations29.
Nitric Oxide Metabolites
In previous papers we and others have demonstrated the ability to up-regulate and/or maintain plasma nitrite during exercise stress is implicated with good vascular endothelial function and increased physical performance in subjects ranging from healthy subjects33, individuals with cardiovascular disease risk factors but no established vascular disease, to those with peripheral arterial disease (figure 3a)17. This suggests, in peripheral arterial disease, under acute exercise stress, endothelial dysfunction may reduce vascular nitric oxide production (and therefore oxidation to plasma nitrite), and coupled with significant vessel stenosis cause greater distal tissue ischemia (claudication pain) which we hypothesized may promote plasma nitrite reconversion back to nitric oxide (for a detailed review of the mechanisms see34).
The primary unique finding of the current paper is that a 3 month SE training regimen resulted in a significant increase in plasma nitrite flux from a negative to a positive balance during an acute cardiopulmonary exercise testing in peripheral arterial disease subjects (figure 3b). This post training response is similar to the comparison control group (without diagnosed cardiovascular disease) at baseline. Interestingly, this increased nitrite flux, associated with an increased ability of the vascular endothelium to respond to the brachial artery flow-mediated dilation testing, suggests a greater capacity to produce nitric oxide to increased blood flow (shear) stimulus (figure 2). This suggests a model in peripheral arterial disease that following 3 months of supervised exercise training produces vascular nitric oxide during normoxia at a rate greater than its consumption by distal hypoxic vascular beds/tissues.
In accordance with the hypothesis of an endocrine-like activity of endothelial derived plasma nitrite is the finding that the Δcardiopulmonary exercise testing plasma nitrite flux and Δclaudication onset pain time following 3 months training are significantly related (figure 4). This relationship was evident for both the peripheral arterial disease supervised exercise and peripheral arterial disease home exercise groups individually, but stronger when the data was combined. This suggests that the balance point for nitrite bioavailability is linked to the point at which subjects experience symptoms of tissue ischemia. Although these findings are supportive of the “push-pull” plasma nitrite-nitric oxide hypothesis, it is currently unclear from this study if an increased endothelial nitric oxide synthase production of (nitric oxide and therefore) plasma nitrite or a greater hypoxic reconversion to nitric oxide (or both) is responsible.
Although our findings using exercise training as a treatment to increase plasma-nitrite flux in peripheral arterial disease, others have recently published studies involving dietary supplementation of nitrate, which results in large increases in plasma nitrite for several hours. These increases were accompanied by decreases in blood pressure35, 36 and possibly oxygen consumption at sub-maximal, but not maximal, exercise workloads37, 38. Clearly, to better define the “push-pull” role on plasma nitrite in peripheral arterial disease exercise performance, further studies are needed that use plasma nitrite supplementation.
Limitations
There are several limitations to our study. This current study is a sub study of a larger peripheral arterial disease exercise study (Angiogenesis and Mechanisms of Exercise Training in PAD -AMNESTI). As such the primary purpose was to examine changes in nitric oxide metabolites and brachial artery flow-mediated dilation to supervised exercise and their relationship to exercise performance. We did not see a significant difference in a primary comparison of supervised exercise vs home exercise in any of the measured parameters. This sub-study was not designed nor statistically powered to answer the question “did the supervised exercise group improve statistically more than the home exercise group?”
A second limitation is that we did not actively control the amount of exercise performed in the home exercise group. The subjects were encouraged to train but this method of implementation has previously been shown to be ineffective for increasing exercise capacity21. Some non-significant increases were seen for all the variables in the home exercise group which is probably due to some of these subjects actually performing the exercise training with no supervision.
From the biochemical perspective, we did not measure the concentration of reactive oxygen species in any of the blood samples. It is likely that the peripheral arterial disease subjects generated greater reactive oxygen species during the cardiopulmonary exercise testing prior to supervised exercise training. This could play a role in the consumption of nitric oxide especially in ischemic tissues. We also did not measure nitric oxide-species on the red cell. It is well documented that nitric oxide is carried on the red cell and can be released during hypoxia39, 40. It has also been shown that plasma nitrite may be converted by deoxyhemoglobin to nitric oxide and iron-nitrosylated hemoglobin12. These mechanisms may also have contributed to the overall nitrite flux.
A fourth limitation to the interpretation of our findings is that we did not measure the oxygen saturation of the working tissues in our subjects during the cardiopulmonary exercise testing. It would be interesting, in future studies, to monitor the relationship between lower limb hemoglobin saturation and plasma nitrite flux during cardiopulmonary exercise testing.
Finally, we also did not report data on changes in skeletal muscle capillary density or oxidative metabolism enzymes which would most likely be increased following training and play a major role in the increased exercise capacities.
SUMMARY/CONCLUSIONS
The results of this study provide an interesting insight into the potential mechanisms of NO and nitrite vascular biochemistry and physiology in peripheral arterial disease. Our earlier work showed the ability of these markers to differentiate clinical disease status and predict VO2peak 17. The current findings extend this work to show improvements in brachial artery flow-mediated dilation, plasma nitrite flux, claudication onset pain time, peak walking time, and VO2peak with supervised exercise training in a peripheral arterial disease population and provide support for the emerging hypothesis that plasma nitrite may function as a stable endocrine carrier and under hypoxic conditions a transducer of nitric oxide-like bioactivity.
Clearly, there is a need for further studies to understand both the biochemical mechanisms involved and the relevance of these mechanisms to vascular physiology and pathology. However the clinical implication of the current findings for pathological conditions where regional ischemia prevails provides an interesting avenue for potential nitric oxide-based therapeutics.
ACKNOWLEDGMENTS
This project was supported by R01 HL755752 from the National Institute of Health, National Heart, Lung, and Blood Institute and the Office of Research on Women’s Health, Office of the Director to BHA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rose G, Blackburn H. Cardiovascular survey methods. Vol 56. Geneva: WHO Monograph Series; 1968. [PubMed] [Google Scholar]
- 2.Belch JJF, Topol EJ, Agnelli G, et al. Critical Issues in Peripheral Arterial Disease Detection and Management: A Call to Action. Arch Intern Med. 2003;163(8):884–892. doi: 10.1001/archinte.163.8.884. April 28, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Verma S, Buchanan MR, Anderson TJ. Endothelial Function Testing as a Biomarker of Vascular Disease. Circulation. 2003;108:2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 4.Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversable impairment of endothelium dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 5.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgapoulos D, Robinson J, Deanfield JE. Aging is assocoated with endothelial dysfunction in healthy men years before the age-related decline in women. Journal of the American College of Cardiology. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 6.Taddei S, Virdis A, Mattei P, et al. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 7.Kunz J. Initial lesions of vascular aging disease (arteriosclerosis) Gerontology. 2000 Nov–Dec;46(6):295–299. doi: 10.1159/000022180. [DOI] [PubMed] [Google Scholar]
- 8.Lauer T, Preik M, Rassaf T, et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. PNAS. 2001;98(22):12814–12819. doi: 10.1073/pnas.221381098. October 23, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen JD, Cobb FR, Gow AJ. Regional and whole-body markers of nitric oxide production following hyperemic stimuli. Free Radical Biology and Medicine. 2005;38(9):1164–1169. doi: 10.1016/j.freeradbiomed.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Kleinbongard P, Dejam A, Lauer T, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radical Biology and Medicine. 2003;35(7):790–796. doi: 10.1016/s0891-5849(03)00406-4. 2003/10/1. [DOI] [PubMed] [Google Scholar]
- 11.Cannon RO, III, Schechter AN, Panza JA, et al. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J. Clin. Invest. 2001;108(2):279–287. doi: 10.1172/JCI12761. July 15, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9(12):1498–1505. doi: 10.1038/nm954. 2003/12//print. [DOI] [PubMed] [Google Scholar]
- 13.Gladwin MT, Shelhamer JH, Schechter AN, et al. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. PNAS. 2000;97(21):11482–11487. doi: 10.1073/pnas.97.21.11482. October 10, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380(6571):221–226. doi: 10.1038/380221a0. 03/21/ [DOI] [PubMed] [Google Scholar]
- 15.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 16.Pinder A, Pittaway E, Morris K, James P. Nitrite directly vasodilates hypoxic vasculature via nitric oxide dependent and independent pathways. British Journal of Pharmacology. 2009;158:1523–1530. doi: 10.1111/j.1476-5381.2009.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen J, Miller E, Schwark E, Robbins J, Duscha B, Annex B. Plasma Nitrite Response and Arterial Reactivity Differentiate Cardiovascular Health Status and Performance. Nitric Oxide Biology and Chemistry. 2009;20:231–237. doi: 10.1016/j.niox.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274(12):975–980. September 27, 1995. [PubMed] [Google Scholar]
- 19.Brendle DC, Joseph LJO, Corretti MC, Gardner AW, Katzel LI. Effects of exercise rehabilitation on endothelial reactivity in older patients with peripheral arterial disease. The American Journal of Cardiology. 2001;87(3):324–329. doi: 10.1016/s0002-9149(00)01367-9. 2001/2/1. [DOI] [PubMed] [Google Scholar]
- 20.Regensteiner J, Steiner J, Hiatt W. Exercise training improves functional status in patients with peripheral arterial disease. J Vasc Surg. 1996;23(1):104–115. doi: 10.1016/s0741-5214(05)80040-0. [DOI] [PubMed] [Google Scholar]
- 21.Stewart K, Hiatt W, Regensteiner J, Hirsch A. Medical Progress: Exercise training for claudication. New England Journal of Medicine. 2002;347:1941–1951. doi: 10.1056/NEJMra021135. [DOI] [PubMed] [Google Scholar]
- 22.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery; A report of the International Brachial Artery Reactivity Task Force. Journal of the American College of Cardiology. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. 2002/1/16. [DOI] [PubMed] [Google Scholar]
- 23.Welsch MA, Allen JD, Geaghan JP. Stability and reproducibility of brachial artery flow-mediated dilation. Medicine and Science in Sports and Exercise. 2002;34(6):960–965. doi: 10.1097/00005768-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Gardner AW, Katzel LI, Sorkin JD, et al. Exercise Rehabilitation Improves Functional Outcomes and Peripheral Circulation in Patients with Intermittent Claudication: A Randomized Controlled Trial. Journal of the American Geriatrics Society. 2001;49(6):755–762. doi: 10.1046/j.1532-5415.2001.49152.x. [DOI] [PubMed] [Google Scholar]
- 25.Gardner AWP, Katzel LIMDP, Sorkin JDMDP, Goldberg APMD. Effects of Long-term Exercise Rehabilitation on Claudication Distances in Patients With Peripheral Arterial Disease: A Randomized Controlled Trial. Journal of Cardiopulmonary Rehabilitation May/June. 2002;22(3):192–198. doi: 10.1097/00008483-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Hiatt W, Regensteiner J, Hargarten M, Wolfel E, Brass E. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation. 1990;81(2):602–609. doi: 10.1161/01.cir.81.2.602. February 1, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Hiatt W, Wolfel E, Meier R, Regensteiner J. Superiority of treadmill walking exercise versus strength training for patients with peripheral arterial disease. Implications for the mechanism of the training response. Circulation. 1994;90(4):1866–1874. doi: 10.1161/01.cir.90.4.1866. October 1, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Patterson RB, Pinto B, Marcus B, Colucci A, Braun T, Roberts M. Value of a supervised exercise program for the therapy of arterial claudication. Journal of Vascular Surgery. 1997;25(2):312–319. doi: 10.1016/s0741-5214(97)70352-5. [DOI] [PubMed] [Google Scholar]
- 29.US-DHHS. Services USDoHaH. Washington DC: U.S. Department of Health and Human Services; 2008. Physical Activity Guidelines Advisory Committee Report, Part G. Section 2: Cardiorespiratory Health 2008. [Google Scholar]
- 30.Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial Dysfunction and cardiovascular Risk Protection in Periperal Arterial Disease: Additive Value of Flow-Mediated Dilation to Ankle-Brachial Pressure Index. Circulation. 2003;108:2093–2098. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- 31.Gokce N, Keaney J, John F, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events inpatients with peripheral vascular disease. Journal of the American College of Cardiology. 2003;41(10):1769–1775. doi: 10.1016/s0735-1097(03)00333-4. 2003/5/21. [DOI] [PubMed] [Google Scholar]
- 32.Payvandi L, Dyer A, McPherson D, et al. Physical activity during daily life and brachial artery flow-mediated dilation in peripheral arterial disease. Vascular Medicine. 2009;14(3):193–201. doi: 10.1177/1358863X08101018. August 1, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rassaf T, Lauer T, Heiss C, et al. Nitric oxide synthase derived plasma nitrite predicts exercise capacity. Br J Sports Med. 2007 doi: 10.1136/bjsm.2007.035758. May 25, 2007 bjsm.2007.035758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundberg JO, Weitzberg E. NO Generation From Nitrite and Its Role in Vascular Control. Arterioscler Thromb Vasc Biol. 2005;25(5):915–922. doi: 10.1161/01.ATV.0000161048.72004.c2. May 1, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. of dietary nitrate on blood pressure in healthy volunteers. New England Journal of Medicine. 2006 Dec;355(26):2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 36.Webb AJ, Patel N, Loukogeorgakis S, et al. Acute Blood Pressure Lowering, Vasoprotective, and Antiplatelet Properties of Dietary Nitrate via Bioconversion to Nitrite. Hypertension. 2008;51(3):784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. March 1, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey SJ, Winyard P, Vanhatalo A, et al. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009 doi: 10.1152/japplphysiol.00722.2009. August 6, 2009 00722.02009. [DOI] [PubMed] [Google Scholar]
- 38.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiologica. 2007;191(1):59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 39.Gladwin MT. Evidence Mounts That Nitrite Contributes to Hypoxic Vasodilation in the Human Circulation. Circulation. 2008;117(5):594–597. doi: 10.1161/CIRCULATIONAHA.107.753897. February 5, 2008. [DOI] [PubMed] [Google Scholar]
- 40.McMahon TJ, Moon RE, Luschinger BP, et al. Nitric oxide in the human respiratory cycle. Nat. Med. 2002;8(7):711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]