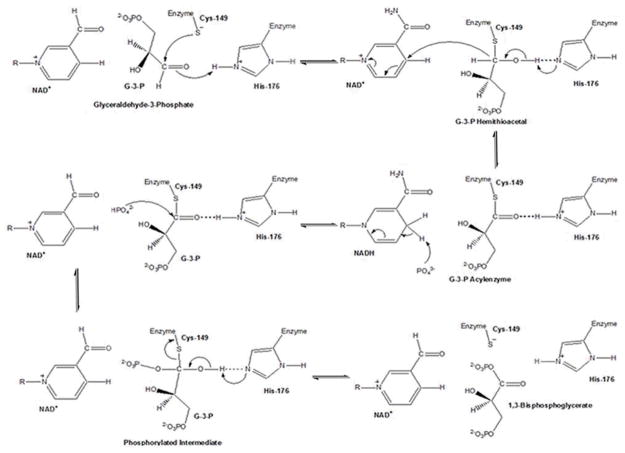
Figure 4. GAPDH Reaction.
The reaction catalyzed by GAPDH involves two processes: Oxidation of the glyceraldehyde-3-phosphate (G3P) aldehyde to a carboxylic acid by NAD+, and joining of the G3P carboxylic acid and the NAD+ orthophosphate to form 1,3-bisphosphoglycerate (BPG). The first step of this mechanism involves nucleophilic attack by the Cys-149 (human analog Cys-152) SH-group on the carbonyl of G3P, resulting in formation of a hemithioacetal. Acting as an acid catalyst, His-176 (human analog His-179) facilitates hemiacetal development by forming an ion pair with the Cys-149 SH-group, enhancing Cys-149 reactivity, and favoring the nucleophilic attack of GAPDH by the thiolate [76]. A hydride transfer to NAD+ oxidizes the hemiacetal intermediate to a high energy thioester, facilitated by His-176, now acting as a base catalyst during the oxidation step [75, 76]. His-176 stabilizes the hemithioacetal intermediate by hydrogen bonding the hemiacetal hydroxyl group to the Nε of its imidazole ring, while the acylenzyme intermediate, is stabilized by hydrogen bonding with the protonated imidazole Nε of His-176 [76]. Finally, the thioester undergoes nucleophilic attack by an inorganic phosphate (Pi), facilitated again by His-176 acting as an acid catalyst, to form BPG [75–77], which is then released from the ternary complex, along with NADH (adapted from [76]).