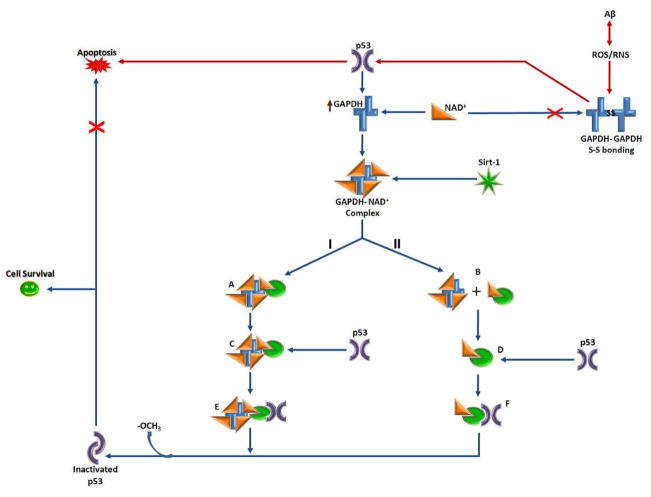
Figure 6. p53 and GAPDH interactions.
This diagram represents possible feedback-loop mechanisms of p53 modulation by GAPDH and the NAD+-dependant protein deacetylase, Sirt-1. Upon binding the GAPDH-NAD+ complex, Sirt-1 undergoes a conformational change that enables it to bind and remove acetyl groups (−OCH3) from C-terminal Lys residues of p53, inactivating it. Inactivated p53 is unable to induce apoptotic processes; thus, this pathway could lead to cell survival (path shown in blue). At present, the nature of the interaction between Sirt-1 and GAPDH unknown; therefore in this diagram, two possibilities are indicated: I, indicates formation of a GAPDH-NAD+-Sirt-1 complex (A), while II, indicates NAD+ transfer from GAPDH, leading to NAD+-Sirt-1 complex formation (B). In either case, the NAD+-Sirt-1 interaction likely changes the conformation of Sirt-1 (C and D), which may further facilitate Sirt-1 interaction with p53 to form complexes E and/or F. Additionaly, oxidative stress in AD causes GAPDH to undergo intermolecular disulfide bonding, inhibiting its activity and ability to bind NAD+, as well as further hindering Sirt-1-induced inactivation of pro-apoptotic p53 (path shown in red).